During the past decade, new therapeutic strategies have been progressively showing a reduction in major adverse cardiac events (MACE). Nevertheless, women with type 2 diabetes (T2D) remain at very high risk of events, not only due to the disease but also because their risk is underestimated, leading to a suboptimal initiation and uptitration of new evidence-based therapies. This risk causes the progressive development of structural cardiac disease and invariably to heart failure (HF) and advanced HF, covering the full spectrum of American Heart Association/American College of Cardiology HF staging.1 This article reviews the main randomised clinical trials (RCTs) that prove the significant cardiovascular (CV) benefits of using new pharmacological treatments, whose implementation in clinical practice would address an urgent need for the reduction of morbidity and mortality in women with T2D.
Prevention
CV events remain high in people with diabetes, especially in women.2 Indeed, recent RCTs report a high rate of MACE (MI, stroke and CV death) under optimal medical therapy (OMT), highlighted by a rate of 9.4–14.9% for 3–4 years follow-up.3–5 To optimise CV prevention in women, three specific periods present themselves as an appropriate time to check clinical status and provide healthy lifestyle advice: when discussing contraceptive choices; before, during and after pregnancy; and during the menopause.
A primary care visit for contraception is a good opportunity to check the clinical status of the patient and to provide key messages promoting healthy habits, including food intake, physical activities, stress management and lifestyle. Information about T2D and its associated risks should be given to help reduce the risk of CV events.
During pregnancy, detection of diabetes is crucial, because gestational diabetes is a strong risk factor for future MACE. A glucose screening test should be performed for every pregnancy. If diabetes is suspected, the diagnosis should be confirmed with a 2-hour glucose tolerance test. Management of gestational diabetes includes a healthy diet, regular exercise (150 minutes per week of moderate-intensity activity) and, if needed, metformin ± insulin with advice and information on preventing episodes of hypoglycaemia. An ultrasound exam between gestational weeks 18 and 20 will detect potential abnormalities in the foetus. Further ultrasound scans at weeks 28, 32 and 36 to monitor the growth of the foetus and the volume of amniotic fluid are recommended, in combination with regular visits from week 38 onwards. The optimal time for delivery is usually considered to be between weeks 38 and 40. After 40 weeks and 6 days, induction of labour or a caesarean are the two medical options to be discussed. Earlier delivery is recommended in cases where diabetes control is poor despite diet and pharmacological options, in order to protect both the baby and the mother.
It is recommended that the baby is fed within 30 minutes of delivery, then every 2–3 hours until the baby’s glycaemic levels become stable.6 The glycaemia of the newborn is tested starting 2–4 hours after birth until it becomes stable. If the glycaemic levels of the newborn are not well controlled, a temporary transfer to the neonatal unit may be required for closer monitoring. After discharge, the baby should have a new glycaemic test 6–12 weeks after birth.
An early test to check new diabetes in a woman who has had gestational diabetes is mandatory due to the increased risk of developing T2D. At 10 years follow-up, after adjustment for ethnicity and age, gestational diabetes is associated with an increased relative risk of CV morbidity between 1.8 and 2.3.7 Therefore, long-term monitoring of women who have had gestational diabetes is highly recommended, with a calcium coronary score performed after the age of 40 to better define the CV risk.
The third crucial period is the transition leading to menopause when the risk of CV events increases whereas management remains poor. Hypertension, dyslipidaemia, obesity and other issues with the metabolic syndrome represent the main risk factors for T2D in the peri-menopausal period, but these remain insufficiently detected and treated in women. Hypertension is a very strong risk factor at this stage and the control of blood pressure is pivotal. Although hormone replacement therapy is effective for decreasing vasomotor symptoms linked to menopause, this option is not recommended due to its negative effects on the CV system.6 Collaboration between GPs, cardiologists and gynaecologists is crucial to better identify high-risk peri-menopausal women. Collaboration centred on the individual woman, with shared decision-making, would provide better detection and long-term management of women with diabetes to prevent MACE.
This call for action for women with diabetes is motivated by the cumulative data that shows they have a very high risk not only for atherosclerotic CV disease (ASCVD) but also for HF, because diabetes causes structural myocardial, coronary and vascular disease, and leads to chronic neurohormonal activation of the angiotensin–aldosterone system and sympathetic nervous system that in the long run is deleterious and causes hydrosaline retention, congestion and translates to a high risk of CV death.8
Optimal management of diabetes in women is challenging due to the under-representation of women in RCTs and registries, highlighting the need of dedicated studies for women (Table 1). Ageing and the high prevalence of comorbidities also represent documented barriers to optimal management. Nevertheless, underestimation of the CV risk and therapeutic inertia contribute to the poorer prognosis of women with diabetes which has been underlined by several registries, where the detection of CV risk is inadequate and women receive fewer recommended treatments with the target dose when compared to men. Recently, new therapeutic classes have emerged which provide additional CV and renal benefits. The development of new classes acting not only on glycaemic control but also by multiple mechanisms already represent valid options to further reduce CV events in women. The modern approach should not only focus only on HbA1c level but much more on a holistic strategy to improve life expectancy and quality of life (QoL).
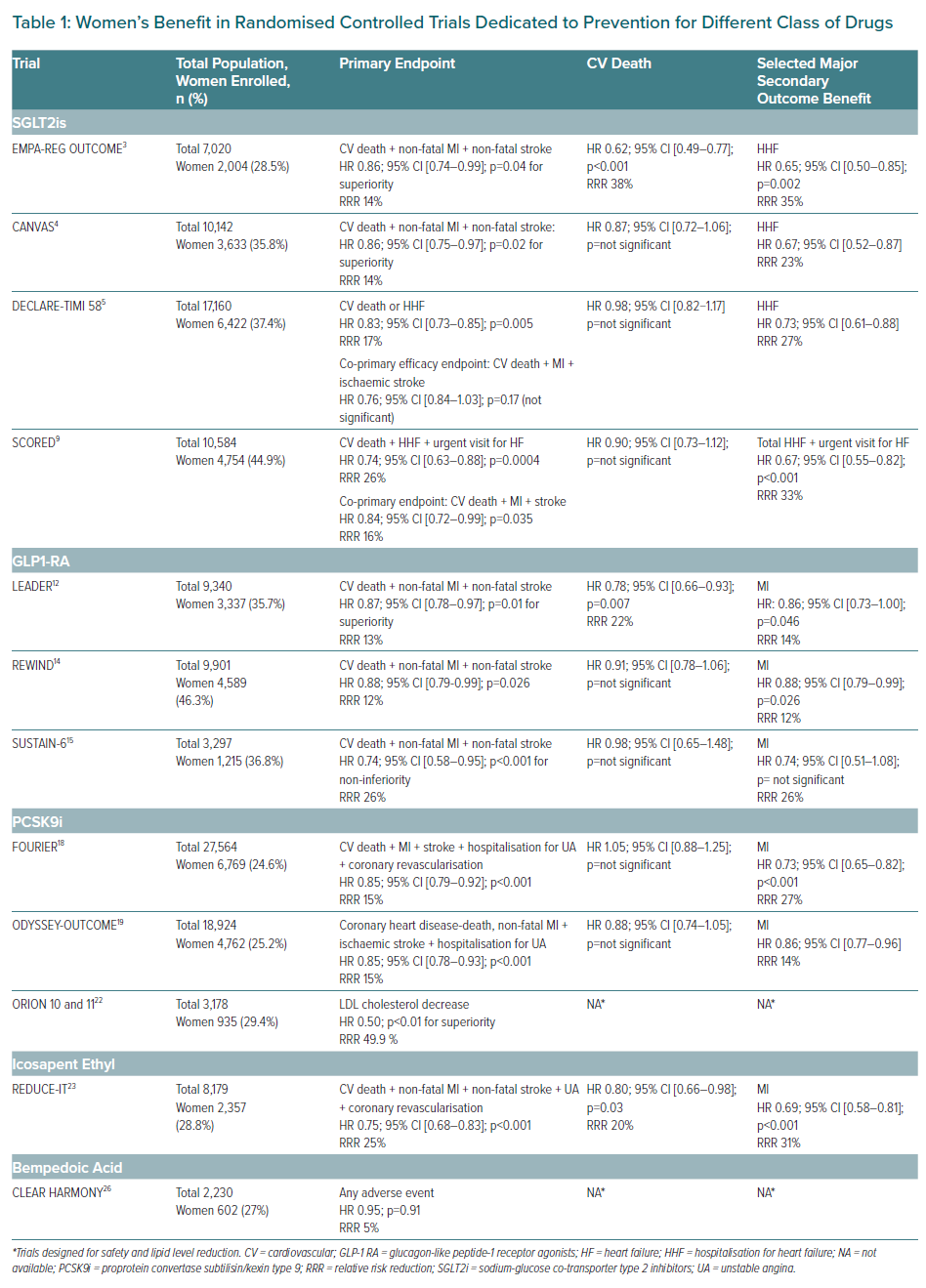
The glucagon-like peptide-1 receptor agonists (GLP-1 RA) are the first class with further CV benefits reported in several RCTs, with heterogeneity between molecules, whereas CV and renal benefits have been proven with several sodium-glucose cotransporter 2 inhibitors (SGLT2i) in EMPA-REG Outcome, EMPEROR-Reduced (empagliflozin), CANVAS Program and CREDENCE (canagliflozin), DECLARE-TIMI 58, DAPA-HF (dapagliflozin), SCORED and SOLOIST-WHF (sotagliflozin), and seem homogenous, even if only sotagliflozin provided a stroke reduction.3–5,9 Once again, women are under-represented in these RCTs, but the reported results were homogenous among groups with no sex differences.
New Anti-diabetic Classes That Act on Glycaemic Levels and Reduce Cardiovascular Events
Sodium–Glucose Cotransporter 2 Inhibitors
SGLT2is act by inhibiting glucose reabsorption in the kidneys, which increases urinary excretion of glucose and allows for better glycaemic control in people with T2D. SGLT2i work independently of insulin regulation and β-cell function. Major RCTs have reported CV benefits and renal protection with five SGLT2is (empagliflozin, canagliflozin, dapagliflozin, ertugliflozin and sotagliflozin). Recent meta-analyses reported that SGLT2is not only reduce the risk of new-onset HF, hospitalisations for HF (HHF), protect from worsening renal function and end-stage renal disease (ESRD), but also reduce all-cause as well as CV death, with similar benefits in patients in primary or secondary prevention and with and without a history of HF.10,11 Moreover, these meta-analyses report a reduction in MI by 25% compared to OMT – with a specific benefit regarding this endpoint proved by canagliflozin in the CREDENCE trial and sotagliflozin in the SCORED trial – without apparent benefits in stroke reduction, except for the recent SCORED trial, requiring further investigation to better understand the underlying mechanisms providing these benefits.9 The magnitude of benefits varied according to baseline CV risk and renal function. Furthermore, two SGLT2i demonstrated efficacy in HF not only in patients with diabetes but also in the pre-specified group of non-diabetic patients.
The same limitations concerning women’s inclusion and sample size were observed and no heterogeneity was found according to sex for this therapeutic class. Therefore, even if specific studies in women are warranted to better define the magnitude of benefits in this major population, it is already crucial that women with diabetes benefit early from these two classes in the therapeutic strategy. Choice of the class should be a shared decision according to the woman’s preferences (one weekly injection versus one daily pill), BMI (as weight loss is more pronounced with GLP-1 RA), HbA1c level (SGLT2is lower HbA11c by 0.4–0.6%; GLP-1 RA by 0.8–1%), renal function, atherothrombotic profile and documented HF. The optimal time to initiate treatment may be discussed, but the strong trend in preventing CV events is to start as soon as possible to decrease the loading of glycaemia, dyslipidaemia and blood pressure.
Glucagon-like Peptide-1 Receptor Agonists
GLP-1 RA work via multiple mechanisms. Their incretin effect – a pleiotropic mechanism – reduces gastric emptying, but mainly acts on plasma glucose modulation through the stimulation of insulin release and reduction of hepatic glucose release (partially mediated by suppressing glucagon secretion). GLP-1 RA initially have been approved for the treatment of T2D because their glucose-lowering effect was associated with a reduction in weight and blood pressure. Liraglutide was the first in class to report CV benefits in the LEADER trial, with a reduction of the primary composite outcome (CV death, MI or stroke) by 13% in the liraglutide group (p<0.001 for non-inferiority; p=0.01 for superiority), a 22% relative RR (RRR) for CV death (p=0.007), and a 15% RRR for all-cause mortality (p=0.02).12,13 Further benefits have been reported in two additional RCTs (REWIND and SUSTAIN-6), with no sex differences observed.14,15 Therefore, European Society of Cardiology (ESC)/ European Association for the Study of Diabetes (EASD) guidelines recommended both classes early in the treatment algorithm, either as monotherapy or in combination with metformin, without sex-related specificities.16 In the setting of HF and/or chronic kidney disease (CKD), SGLT2is provide more benefits than GLP-1RA and are the preferred option for women with diabetes.17
New Therapies Providing Benefits in Women with Diabetes Outside Glucose Metabolism
Proprotein Convertase Subtilisin/Kexin Type 9 Inhibitors and Inclisiran
These two classes of drugs inhibit the proprotein convertase subtilisin/kexin type 9 (PCSK9), which modulates the cholesterol level, mainly by reducing the numbers of LDL receptors on the plasma membrane.
Two RCTs evaluating PCSK9 inhibitors (FOURIER and ODYSSEY Outcomes) reported CV benefits for a short-term follow-up (about 2 years), one in ASCVD patients including chronic coronary stable patients, peripheral artery disease and people who have had a stroke, the other in patients with post-acute coronary syndromes.18–20 In patients with LDL cholesterol levels of 1.8 mmol/l or higher under maximal tolerated dose of statin therapy, both PCSK9is reduce the primary combined endpoint with a RRR of 15%, without a warning signal on safety even for very low LDL cholesterol levels. As the CV risk was higher in people with diabetes, the clinical benefits of PCSK9i were more pronounced without sex differences. Glycaemic parameters remained unchanged with either PCSK9is.
Inclisiran is a small interfering RNA molecule, which targets the hepatic production of PCSK9. Inclisiran induces gene silencing through repression of transcription of specific genes, promoting cleavage and elimination of PCSK9, and reduces LDL cholesterol by 50–70% without any safety signals to date. This safety and biological efficacy were first observed in Phase II clinical trials, and the on-going RCT Phase III ORION 4 (NCT03705234) is evaluating the clinical efficacy of inclisiran versus placebo in ASCVD patients, including women with diabetes.21 The results are expected in 2025.
Icosapent Ethyl
Icosapent ethyl (EPA) is a purified eicosapentaenoic acid formulation which reduces hepatic production and secretion of very low-density lipoproteins, increasing triglycerides clearance without major safety concerns. The clinical efficacy has been proven in the REDUCE-IT trial, which enrolled ASCVD patients (70.7%) or patients with diabetes and associated CV risk factors.22,23 Almost all patients (99.5%) were on statin therapy with a triglyceride level of 3.5–12.9 mmol/l at inclusion, and 8,179 patients (2,357 women) were randomised to 4 g/day of EPA or placebo. EPA reduced the primary composite endpoint (CV death, non-fatal MI, non-fatal stroke, coronary revascularisation or unstable angina) by 25% RRR (p<0.0001) after a mean follow-up of 4.9 years. The clinical efficacy of EPA was more pronounced in people with diabetes without sex differences, and seems to be unrelated either to achieved triglycerides or LDL cholesterol levels, suggesting other underlying mechanisms. As in Phase II trials, no major safety issues were reported, except a slight increased risk of hospitalisation for AF (3.1% versus 2.1%; p=0.004). EPA therefore represents an option to reduce MACE in women with diabetes following the National Lipid Association statement.24
Bempedoic Acid
Bempedoic acid (ETC-1002) inhibits adenosine triphosphate citrate lyase, which decreases cholesterol production in the liver. The decreased cholesterol synthesis promotes LDL receptor upregulation and thereby decreases plasma LDL cholesterol. ETC-1002 is converted to its active moiety in the liver by the very long-chain acyl-CoA synthetase-1, an enzyme absent in skeletal muscle, which explains why ETC-1002 is not associated with muscle symptoms and can be particularly useful in patients with statin intolerance.25
In summary, prevention in women with T2D remains suboptimal and may be improved by combining these novel therapeutic strategies with non-pharmacological measures. All these pharmacological agents have proven clinical efficacy without serious safety issues. Further efforts are needed to promote widespread use of these strategies for women, who are frequently under-treated despite recent educational campaigns to better assess and manage their CV risk. Dedicated studies in women with diabetes are warranted.
Heart Failure Management
Even if good preventive therapies are started, T2D patients who are in Stage A maintain a high risk of developing structural heart diseases (Stage B) and progressing to overt HF (Stages C and D).1 As shown by a large number of registries and meta-analyses including millions of patients, this portends a risk of 5-year mortality higher than many cancers and an extremely low QoL, influenced by the high rate of rehospitalisation and by symptoms and reduced activity as measured by several dedicated questionnaires, such as the Kansas City Cardiomyopathy Questionnaire (KCCQ).26–29 The major impact of HF development is even more ominous in women. Indeed, as recently highlighted by reviews and meta-analyses, women receive later referrals to HF treatment centres compared with men, are under-enrolled in dedicated HF RCTs, receive cardiac resynchronisation therapy/ICD and left ventricular assist device/heart transplant less frequently, have higher mortality on the heart transplant waiting list and have worse QoL.30,31
Despite HF being more frequent in women than in men, women are systematically under-enrolled in RCTs so that the specific benefits of HF drugs are less well known.32 In fact, women represent a specific population in which not only the exact dosages (that can be lower or equal), but also the pathophysiological pathways (that can be involved with different magnitude or can even be different) of HF drugs are less studied and understood because women have been treated as ‘smaller men’ which is evidently wrong. RCTs dedicated to HF usually enrol a maximum of 30% of women – at least in part due to under-enrolment of older patients, a higher percentage of whom are women. We strongly hope that will change and we encourage researchers to conduct dedicated RCTs for women or to enrol higher percentages of women to fill our knowledge gap. On the other hand, T2D patients are well represented in some of the most recent HF RCTs (ranging from 34.9–49.8%), reflecting the percentage of T2D in HF reported by large clinical registries (about 40%).33,34
After more than 30 years of triple therapy – angiotensin-converting enzyme inhibitors (ACEi) or angiotensin receptor blocker (ARB), β-blockers and mineralocorticoid receptor antagonists (MRA) – ivabradine represented a first, small improvement in reducing HHF and HF death without any improvements on CV death and all-cause death.35 The real revolution in HF with reduced ejection fraction (HFrEF) therapy started in 2014 with PARADIGM-HF, which proved a further reduction of major outcomes using the angiotensin receptor neprilysin inhibitor (ARNI) sacubitril/valsartan compared with the ACEi enalapril, and continued from 2019 to now with the new class of drugs SGLT2is and with the new drugs vericiguat and omecamtiv mecarbil.36
SGLT2is: The New Pillar of Cardiorenal Benefit in HF and Chronic Kidney Disease
As previously mentioned, meta-analyses of RCTs dedicated to prevention in T2D have demonstrated a significant benefit on CV death and MACE, but the magnitude of benefit was unexpectedly greater on the endpoints of CV death and HHF, and significant for each individual RCT and not only in the pooled analysis.10
Chronic Heart Failure with Reduced Ejection Fraction
Noticing this evident benefit, all the developers of SGLT2is designed RCTs exclusively dedicated to HF patients with and without T2D, such as EMPEROR-Reduced (empagliflozin in HFrEF), EMPEROR-Preserved (empagliflozin in heart failure with preserved ejection fraction [HFpEF]), CHIEF-HF (canagliflozin in both HFrEF and HFpEF), DAPA-HF (dapagliflozin in HFrEF), DELIVER (dapagliflozin in HFpEF) and SOLOIST-WHF (sotagliflozin in AHF).38,41,44,50
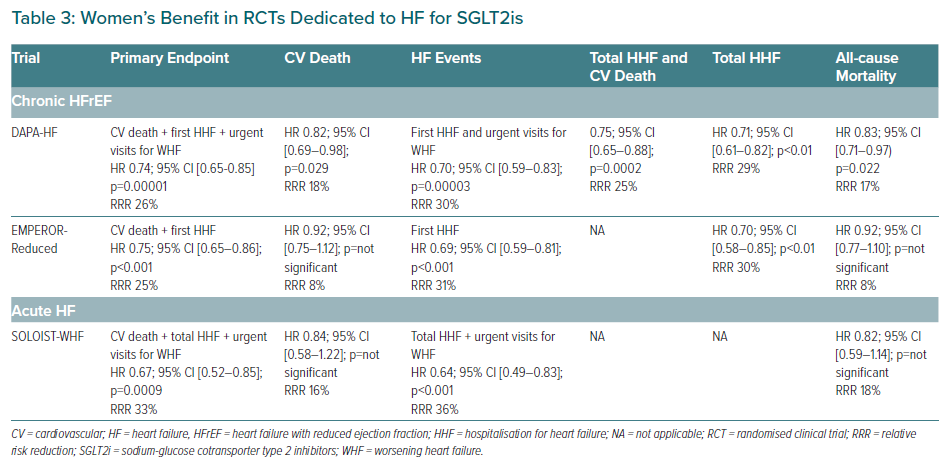
The first published RCT was DAPA-HF on dapagliflozin in HFrEF.37 DAPA-HF enrolled and randomised 4,744 patients with the following characteristics mean age 66.4 years, 23% women, T2D 41.8%, New York Heart Association (NYHA) stage II 67.5%, median N-terminal pro-B type natriuretic peptide (NT-pro-BNP) was 1,437 pg/ml, mean estimated glomerular filtration rate (eGFR) was 66 ml/min/1.73 m2, 40.2% had eGFR <60 ml/min/1.73 m2. Key inclusion criteria are shown in Supplementary Material Table 1 and detailed demographic and clinical characteristics are shown in Table 2. Considering OMT, patients were well treated: ACEi/ARB/ARNI pooled 94% (split: ACEi 56%, ARB 28%, ARNI 11%), β-blockers 96%, MRA 71%, ivabradine 5%. The OMT percentages of DAPA-HF were even better than that of PARADIGM-HF. Noticeably, as in PARADIGM-HF, the percentages of CRT/ICD were particularly low in DAPA-HF (7% and 26%, respectively), showing the intention to test the efficacy of the drug before the implantation of any devices (Table 2).
After a mean follow-up of 18.2 months, dapagliflozin 10 mg/day versus placebo significantly reduced the primary endpoint: a composite of CV death and first HHF and urgent visit for worsening HF (WHF; (HR 0.74; 95% CI [0.65–0.85] p=0.00001) with a RRR of 27% and a number needed to treat (NNT) of 21. Note that WHF is considered an equivalent of HHF in the last ESC criteria for advanced HF. See Table 3 for the effect on other major outcomes.38 Beyond the remarkable results achieved in reducing CV death and all-cause death, the secondary endpoint dedicated to QoL in HFrEF was significantly reduced, as demonstrated by the improvement in the total symptoms score of the KCCQ.39 In DAPA-HF there were no differences regarding the incidence of the secondary endpoint ‘worsening renal function’ – a composite of sustained ≥50% reduction in eGFR, ESRD or death from renal causes (HR 0.71; 95% CI [0.44–1.16]; p=0.17).
By far the most remarkable result has been the pre-specified subgroup analyses, which did not show any difference regarding the reduction of primary endpoint between diabetic patients (HR 0.75; 95% CI [0.63–0.90]) and non-diabetic patients (HR 0.73; 95% CI [0.60–0.88]). In particular, non-diabetic patients had a RRR of 27% for the primary endpoint, representing the real and unexpected result of DAPA-HF. Importantly, there was no difference between the magnitude of reduction of the primary endpoint in patients with eGFR >60 versus <60 ml/min/1.73m2. Consistently, dapagliflozin did not signal safety concerns, indeed, there were no significant differences versus placebo in terms of volume depletion, renal adverse events, fractures, limb amputations, major hypoglycaemia (also shown in patients without T2D), diabetic ketoacidosis, urinary infections or all infections; whereas any serious adverse events were significantly higher in the placebo than in the dapagliflozin group. These results represented a breakthrough in HFrEF management because dapagliflozin provided additional benefits on top of OMT (including 11% of patients on ARNI) irrespective of diabetic status and this is also valid for women.
The second RCT published on HF was EMPEROR-Reduced, dedicated to empagliflozin in HFrEF.40 EMPEROR-Reduced enrolled and randomised 3,730 patients with a similar design to DAPA-HF. However, EMPEROR-Reduced studied a population with a more compromised renal function (mean eGFR 62 ml/min/1.7m2, 48.3% had eGFR<60 ml/min/1.73 m2) and a more severe HFrEF, characterised by a lower mean LVEF (27.5%), higher levels of median NT-pro-BNP (1,906 pg/ml), and an annual placebo event rate of 20% (compared to the 15% of the population enrolled in DAPA-HF) despite a better OMT, i.e. a greater percentage of patients in ARNI (19.5%; Table 2). After a mean follow-up of 16 months, empagliflozin 10 mg/day versus placebo on top of HFrEF standard of care, significantly reduced (HR 0.75; 95% CI [0.65–0.86]; p<0.001) the primary endpoint – a composite of CV death and first HHF with a RRR of 25% and an NNT of 19 – the first hierarchical secondary endpoint (total HHF; Table 3) and significantly improved the second hierarchical secondary endpoint, the mean slope of change in eGFR (HR 1.3; 95% CI [1.10–2.37] p<0.001). The renal benefit has also proved by the reduction of the composite renal endpoint (ESRD + sustained profound eGFR decrease) (HR 0.50; 95% CI [0.32–0.77]) with a RRR of 50%.
Beyond the results achieved on primary and secondary endpoints, empagliflozin also showed a significant benefit on QoL in HFrEF measured by the symptoms score of the KCCQ that was significantly better than placebo (p=0.0058; absolute difference 1.7). As in DAPA-HF, the prespecified subgroup analyses did not show any significant difference regarding the reduction or primary endpoint between diabetic patients (HR 0.72; 95% CI [0.60–0.87]; RRR 28%) and non-diabetic patients (HR 0.78;95% CI [0.64–0.97]; RRR 22%) and between patients with eGFR>60 versus <60 ml/min/1.73 m2. Empagliflozin showed the same safety profile dapagliflozin in HFrEF, especially regarding volume depletion, hypotension, bone fractures, limb amputations, severe hypoglycaemia (also in patients without T2D), diabetic ketoacidosis and urinary infections. As in the RCTs dedicated to prevention, empagliflozin significantly increased genital infections compared to placebo, but these represented only 1.7% of patients in the empagliflozin arm (1,863); this data is not available for DAPA-HF.
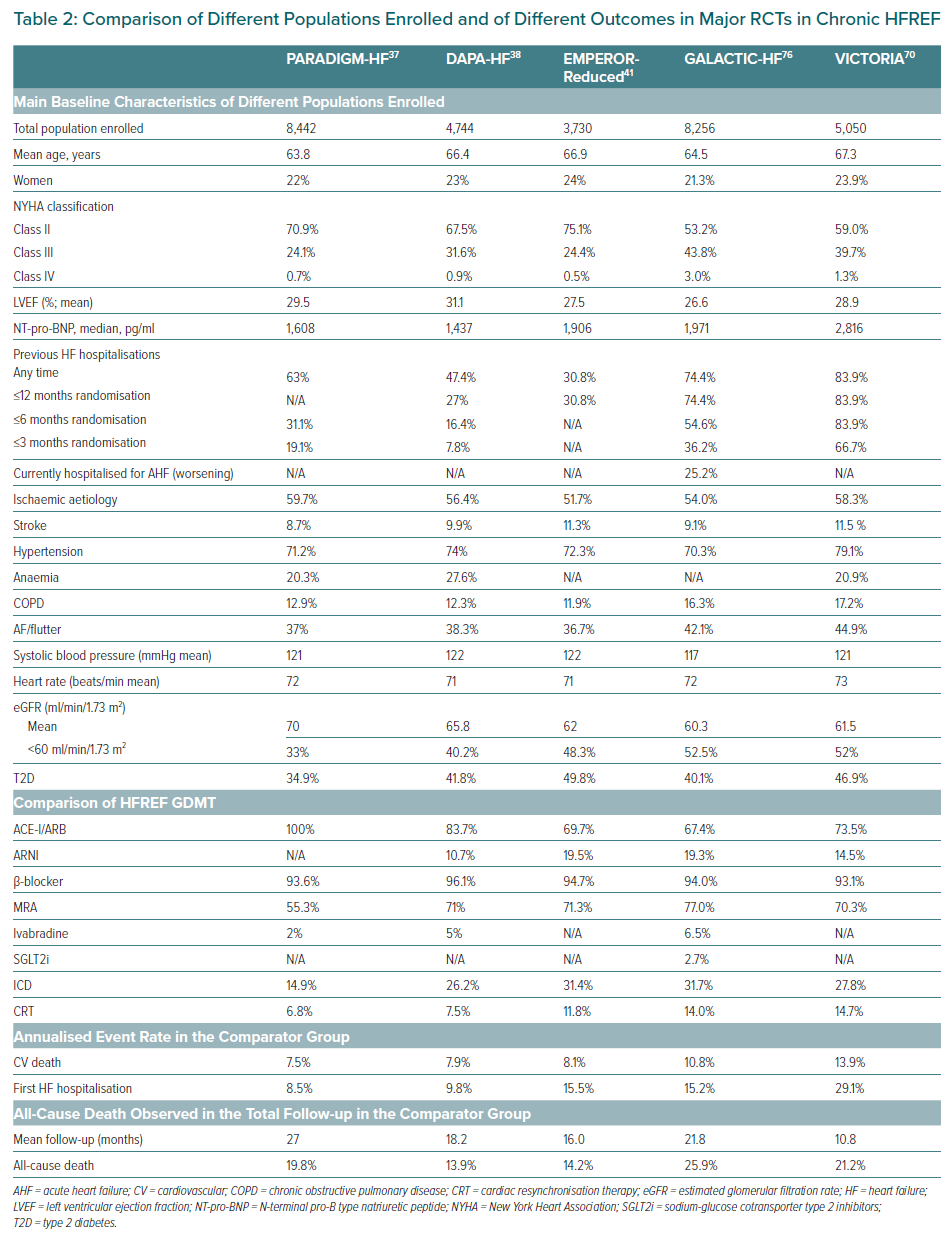
Finally, in EMPEROR-Reduced, CV death and all-cause death were not significantly reduced, so some authors have speculated about the impact of empagliflozin on mortality. The reasons of the lack of statistical significance have been elegantly discussed in a recent editorial.41 Previously, we mentioned that the population enrolled in EMPEROR-Reduced had a higher annual placebo event rate of the primary endpoint when compared to the population of DAPA-HF (24.7 versus 15.3 per 100 person-years). A further analysis of the placebo event rate showed that the rate of CV death was nearly the same between the two RCTs (8.1 versus 7.9 per 100 person-years in the placebo group for EMPEROR-Reduced and for DAPA-HF, respectively) whereas the rate of first HHF of EMPEROR-Reduced was significantly higher than in DAPA-HF (15.5 versus 9.8 per 100 person-years; Table 2). This shows that in EMPEROR-Reduced the higher primary endpoint rate was driven by an elevated rate of HHF but not rate of death, and this higher primary endpoint rate led to a shorter mean follow-up of this RCT when compared to DAPA-HF (16.0 versus 18.2 months). On the other side, the sample size of patients enrolled in EMPEROR-Reduced was significantly smaller than in DAPA-HF (3,730 versus 4,744), this had the final effect to significantly reduce the statistical power of the RCT. Indeed, there were 111 fewer CV deaths and 90 fewer all-cause deaths in EMPEROR-Reduced than in DAPA-HF (389 versus 500 and 515 versus 605).
A further element aggravating this reduced statistical power on mortality was the higher study treatment discontinuation rate in EMPEROR-Reduced, compared with DAPA-HF (17.1% versus 10.7%). Considering the significant reduction of CV death in EMPAREG-OUTCOME that was not seen in DECLARE-TIMI58, it is very likely that both drugs have the same effect in reducing CV death in T2D and in HFrEF, and the differences outlined above are related to the design and conduction of each trial. EMPEROR-Reduced demonstrated the efficacy of SGLT2i on a more severe HFrEF population and provided complementary data to those of DAPA-HF, strengthening the evidence of benefit of this class of drugs in HF.
The first meta-analysis dedicated to SGLT2i in HFrEF showed that when considered together (empagliflozin and dapagliflozin) consistently reduce CV death (p=0.027 for efficacy; HR 0.86; 95% CI [0.76–0.98]; RRR 14%; p=0.39 for heterogeneity), all-cause death (p=0.018 for efficacy; HR 0.87 95% CI [0.77–0.98]; RRR 13%; p=0.39 for heterogeneity), CV death and first HHF (p<0.0001 for efficacy; HR 0.74; 95% CI [0.68–0.82]; RRR 26%; p=0.89 for heterogeneity), CV death and total HHF (p<0.0001 for efficacy; HR 0.75; 95% CI [0.68–0.84]; RRR 25%; p=0.91 for heterogeneity), first kidney composite outcome (p=0.013 for efficacy; HR 0.62; 95% CI [0.43–0.90]; RRR 38%; p=0.42 for heterogeneity). Furthermore, the effect on the primary endpoint is independent of the fact that patients had T2D, were receiving ARNI or had an eGFR >60 versus <60 ml/min/1.73 m2.42
Acute Heart Failure
The other major RCT in HF is SOLOIST-WHF dedicated to sotagliflozin (an SGLT1i and SGLT2i) which enrolled a population with AHF.43 Following the encouraging results of EMPA-RESPONSE-AHF (a pilot study on empagliflozin in very early phases of AHF), SOLOIST-WHF enrolled 1,222 patients with the following characteristics: median age 70 years; 33.7% women; NYHA II 45.2%, NYHA III 45.8% and NYHA IV 4.4%; median LVEF 35% (79.1% had an LVEF <50%, 20.9%, LVEF ≥50%); median HbA1c 7.1%; median NT-pro-BNP was 1,799.7 pg/ml; median eGFR: 49.7 ml/min/1.73 m2; and 47.1% had a history of AF (key inclusion criteria in Supplementary Material Table 1).44 Patients were very well treated: ACEi/ARB/ARNI pooled 99.4% (ACEi: 40.5%, ARB: 42.1%, ARNi: 16.8%), β-blockers 92.1%, MRA 64.5%, loop-diuretic 95%, CRT/ICD 20.3%, any glucose-lowering medications (metformin/insulin/GLP1-RA/dipeptidyl peptidase-4 inhibitors/sulfonylurea): 85.4%. Sotagliflozin could be started prior to discharge from the hospital until a maximum of 3 days post-discharge. Half of the patients started the drug during HHF. After a median follow-up of 9.2 months, sotagliflozin 200 mg/day (uptitrated to 400 mg/day as tolerated) versus placebo on top of HF treatments significantly reduced the primary endpoint, a composite of CV death + total HHF + urgent visits for WHF, with a RRR of 33% and NNT of 4 (HR 0.67; 95% CI [0.52–0.85]; p=0.0009). The first secondary hierarchical endpoint: total HHF + urgent visits for WHF was significantly reduced whereas CV death (the second secondary hierarchical endpoint) considered alone as well as all-cause death were not significantly reduced (Table 3).
Beyond the endpoints on major outcomes, sotagliflozin significantly improved QoL as reported by a 4.1 point increase in KCCQ (p=0.005) and showed a significant renal benefit confirmed by the lower mean reduction in the eGFR compared to placebo after the initial treatment period (p=0.02). Similar to DAPA-HF and EMPEROR-Reduced, there were no major safety issues with sotagliflozin, with the difference of significant increase of diarrhoea (6.9% versus 4.1%) and severe hypoglycaemia (1.5% versus 0.3%) with respect to placebo.
SOLOIST-WHF was the first RCT in the history of cardiology to demonstrate a significant reduction of major combined endpoints such as total HHF + CV death + urgent visits for WHF. Despite that, CV death, as well as all-cause death, have not been significantly reduced when considered alone, but the same considerations for EMPEROR-Reduced are even more valid for SOLOIST-WHF which was largely underpowered for mortality. Indeed, loss of funding from the sponsor during enrolment – also affected by the coronavirus disease 2019 pandemic – led to the trial enrolling only 1,222 of the planned 4,000 patients and it being stopped earlier than planned.
The major conclusion is that in AHF an initiation of an SGLT2i in a late phase of hospitalisation provided significant benefits, and this is just the beginning of evidence because RCTs with a similar design such as EMPULSE (empagliflozin) and DAPA ACT HF-TIMI68 are ongoing, while DICTATE-AHF is enrolling patients with AHF who will start dapagliflozin in the early phase of HHF.45
Heart Failure with Preserved Ejection Fraction
HFpEF is a multifaceted syndrome and over the past 20 years, several efforts have been made to identify, recognise and characterise this complex syndrome that under this general label hides dozens of different diseases ranging from hypertensive heart disease, obesity, metabolic syndrome, pulmonary hypertension to cardiac amyloidosis, hypertrophic cardiomyopathy and restrictive cardiomyopathies.46
Several studies and registries showed that HFrEF and HFpEF number or comorbidities are very similar and their number is related to the patient’s age. 33,34 Furthermore, HFpEF due to its kaleidoscopic nature had several problems of definition so that recently the Heart Failure Association of the ESC redefined the diagnostic criteria to reduce the possibility of misdiagnosis.47 However, the complexity of HFpEF has never been correctly assessed over time and consequently a large number of molecules have been tested in RCTs by the ‘all-comers’ or ‘one-size-fits-all’ approach, with several failures. As a major consequence, no therapies have been shown to convincingly modify prognosis in HFpEF.
This has pushed researchers to better identify specific phenotypes and to develop specific therapies for different phenotypes or different aetiologies of HFpEF. One of the clearly identified phenotypes is constituted by women with the metabolic syndrome (or even T2D) who develop concentric hypertrophy without chamber dilatation and dominant diastolic dysfunction.48
In this area, pooled data from SOLOIST + SCORED in HFpEF patients were presented. The opportunity given by this analysis is to have a larger sample size (one derived from an RCT dedicated to chronic kidney disease and the other one to HF, constituted by 739 HFpEF patients. Sotagliflozin significantly reduced the endpoint total CV death, HHF and urgent heart failure visits, with RRR 37% (HR 0.63; 95% CI [0.45–0.89]; p=0.009). The same happened with the 456 HFmrEF, with RRR 39% (HR 0.61; 95% CI [0.40–0.94]).
A press release on EMPEROR-Preserved (a dedicated RCT of empagliflozin in HFmREF and HFpEF) revealed that empagliflozin has met the primary endpoint of CV death and HHF (Supplementary Material Table 1).49 The reduction of the primary endpoint is significant, so that this is the first RCT in the history of cardiology that has a significant impact on prognosis in this population. Moreover, this will confirm that SGLT2is have multiple benefits in HF irrespective of LVEF.
Chronic Kidney Disease
As mentioned above, meta-analyses of RCTs dedicated to prevention in T2D provided another unexpected benefit – the significant reduction (both for each RCT and for the pooled analysis) of the composite endpoint: worsening renal function + ESRD + renal death not only in patients with an established ASCVD but also in T2D patients without ASCVD.10 Equal to what happened for HF, the developers of these drugs designed and conducted RCTs dedicated to patients with CKD, such as CREDENCE, DAPA-CKD, and EMPA-KIDNEY, the results of the first two RCTs are already published and a detailed discussion of these trials is outside of the purpose of this review.50,51 In summary, CREDENCE – dedicated to canagliflozin 100 mg/day in CKD – enrolled 4,401 patients with the following main characteristics: mean age 63 years, 33.9% women, mean eGFR 56.2 ml/min/1.73 m2 (key inclusion criteria in Supplementary Material Table 1).50 DAPA-CKD – dedicated to dapagliflozin 10 mg/day in CKD – enrolled 4,304 patients with the following main characteristics: mean age 61.9 years, 33.1% women, mean eGFR 43 ml/min/1.73 m2, 67.5% T2D, 32.5% without T2D (key inclusion criteria in Supplementary Material Table 1).51 In short, these two RCTs showed a significant reduction in the same primary endpoint – a composite of ESRD, doubling of serum creatinine, renal death, and CV death. All the secondary endpoints were significantly reduced as well, both for CREDENCE (CV death + first HHF, MACE, first HHF) and for DAPA-CKD (ESRD + renal-death + ≥50% sustained eGFR decline, CV death + first HHF, all-cause death). The most important result is probably that of DAPA-CKD because this was achieved irrespective of diabetic status.
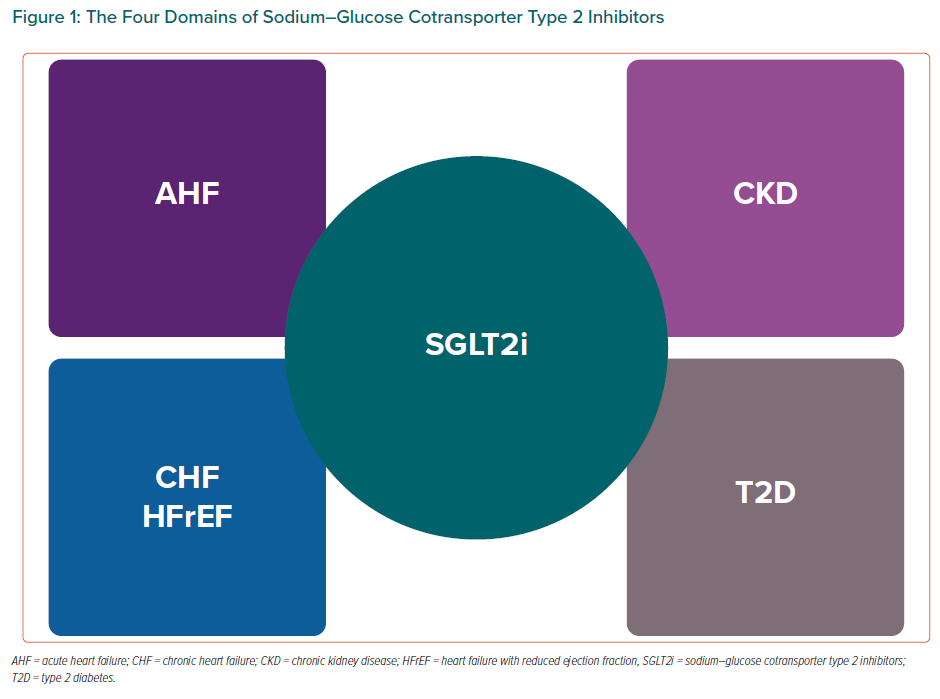
In conclusion, we can state that the four domains of SGLT2is are: T2D, CHF, AHF and CKD (Figure 1) and after the publication of EMPEROR-Preserved we will probably be able to add a fifth domain of HFpEF.
Recently, we published a review in which we discussed the multiple mechanisms of cardiorenal benefits of this class of drugs with respect to the previous review we underline a recent discovery of a new mechanism that can be highly beneficial particularly to women.52,53 Indeed, two separate research groups were able to demonstrate (in human, mouse and pig models) that SGLT2i activate, both in HFrEF and in HFpEF, the pathway of nitric oxide (NO)/cyclic guanosine monophosphate (cGMP)/protein kinase G (PKG) that provokes the titin phosphorylation and ends in reducing cardiomyocyte stiffness and interstitial myocardial fibrosis, with particular benefit on diastolic function.54,55 Moreover, this pathway has important effects on vessel function and remodelling (see vericiguat) and this can be highly beneficial in particular for women that are older than men at HF diagnosis and have a more compromised vascular function.
Another important clue to underline is a recent subanalysis of DAPA-HF, which demonstrated that SGLT2is cause a ‘smart’ reduction of systolic blood pressure (SBP).56 In fact, they reduce SBP only in patients with hypertension but in hypotensive patients (SBP ≤110 mmHg) they do not have any impact on blood pressure. This is a particular advantage in specific groups of HFrEF patients who have low SBP and cannot tolerate ACEi/ARB/ARNI or high doses of β-blockers. Women in clinical practice are more exposed and often present with lower SBP than men so drugs with minimal or no impact on that offer a specific practical advantage.
Sacubitril/Valsartan: A Drug with Potential Higher Impact on Women
Chronic Heart Failure with Reduced Ejection Fraction and Acute Heart Failure
The multiple benefits of the ARNI sacubitril/valsartan have been well established since 2014 when PARADIGM-HF was published.36 The design of this RCT is similar to DAPA-HF. PARADIGM-HF is the largest RCT by population enrolled in the history of HFrEF so far, enrolling 8,442 patients, 22% of whom were women. The major clinical and demographic characteristics are summarised in Table 2. After a mean follow-up of 27 months, sacubitril/valsartan (uptitrated to 200 mg twice a day) versus enalapril on top of HFrEF standard of care, significantly reduced the primary endpoint: a composite of CV death and first HHF (HR 0.80; 95% CI [0.73–0.87]; p<0.0001), with RRR 20% and NNT 21, as well as the split separate components of the primary endpoint, i.e. CV death (HR 0.80; 95% CI [0.71–0.89]; p<0.001, RRR 20%) and the first HHF (HR 0.79; 95% CI [0.71–0.89]; p<0.001, RRR 21%].36 Also, the secondary endpoint all-cause death has been significantly reduced (HR 0.84; 95% CI [0.76–0.93]; p=0.0009, RRR 16%). Beyond this major outcome, sacubitril/valsartan compared to enalapril significantly reduced sudden cardiac death, total HHF, total hospitalisations, as well as total emergency department admissions for WHF, total stays in the intensive care unit and significantly improved symptoms (measured by NYHA) and QoL measured by KCCQ. A detailed analysis of PARADIGM-HF is outside of the scope of this review, but after this RCT, ARNI have been recommended globally from all the international HF guidelines in substitution of an ACEi/ARB in chronic HFrEF as a class I indication.
The RCTs TITRATION, PIONEER-HF and TRANSITION extensively proved the safety of this drug when compared to an ACEi both in the chronic ambulatory HFrEF setting and in the AHF hospitalised setting in terms of worsening renal function, renal adverse events, hyperkalaemia and angioedema.57–59 Hypotension and symptomatic hypotension are more frequent than with ACEi/ARB, but in those RCTs this did not lead to drug discontinuation and has been managed with lowering ARNI dosage. A slower uptitration regimen – as TITRATION showed – reduces the incidence of hypotension and this is even more useful in women with HFrEF who often have lower blood pressure in the clinical scenario.57
PIONEER-HF and TITRATION showed important benefits in terms of efficacy in key secondary exploratory endpoints (CV death and HHF), so that they are suggested in recent ACC and CCS guidelines as first-line treatments in the AHF setting. PROVE-HF and EVALUATE-HF proved that ARNI improve cardiac remodelling in terms of ventricular volume reduction and systolic function improvement. More trials (PARADISE-MI and LIFE) were presented at the American College of Cardiology’s 2021 scientific session.60,61
Heart Failure with Mid-range (Mildly Reduced) and Preserved Ejection Fraction
The most important RCT that showed particular benefit for women is PARAGON-HF, dedicated to sacubitril/valsartan versus valsartan in HFpEF.62 PARAGON-HF enrolled 4,822 HF patients with an LVEF >45% (indicating a mix of HFmrEF and HFpEF), NYHA class II–IV, elevated level of natriuretic peptides (with different cut-offs depending on the occurrence of a recent HHF and the presence of AF), evidence of structural heart disease, and on diuretic therapy (Supplementary Material Table 1). Noticeably, 51.7% of patients were women, NYHA class II 77.7% and NYHA class III 19.4%. After a median follow-up of 35 months, PARAGON-HF failed to demonstrate a significant reduction of the primary endpoint of total HHF + CV death (HR 0.87; 95% CI [0.75–1.01]; p=0.059; RRR 13%). The reasons for this lack of efficacy have been explored in several papers and discussed in heated debates, but invariably lie in the elevated number of exclusion criteria (with 5,537 patients excluded during the screening phase) who made the population of PARAGON-HF far from the real population of HFpEF patients, the all-comers approach (that had already proved to be disastrous in HFpEF), and a likely wrong primary endpoint that, unlike the DAPA-HF, did not include urgent visits for HF (a recent post-hoc analysis showed that in this case, the reduction of primary endpoint would have been significant).63–65 Interestingly, prespecified analyses, showed a significant benefit for two specific subgroups – HF with an LVEF <57% (substantially the HFmrEF population; HR 0.78; 95% CI [0.64–0.95]; p=0.03 for interaction) and women (HR 0.73; 95% CI [0.59–0.90]; p=0.017 for interaction).
The women in the trial were older than men, had more HF symptoms (indicated by worsening NYHA class), a lower median NT-pro-BNP level, worse QoL measured by KCCQ, higher median LVEF (60% versus 55%) than men. Moreover, women had a lower mean eGFR, had a greater incidence of obesity, had less coronary heart disease, T2D, and chronic obstructive pulmonary disease. More detailed analysis showed that the higher benefit of ARNI in women than in men was covered mostly by HHF with RRR of 33% (HR 0.67; 95% CI [0.54–0.84]; p=0.0048 for interaction), without reduction in CV death (HR 1.05; 95% CI [0.78–1.41], p=0.37 for interaction). The improvement in NYHA class was similar in women and men, whereas the improvement in KCCQ seemed to be lower in women than in men.66
These results can be explained by the activation of different pathways in women or more likely by the activation of the same pathways but with different strength and intensity in women than in men. Indeed, despite worse symptoms and QoL, the lower median level of natriuretic peptides in women enrolled in PARAGON-HF was mostly driven by a higher prevalence of obesity and NT-pro-BNP is just a marker and the non-active part of the effective hormones (ANP/BNP). Other studies coming from a subanalysis of PROVE-HF have demonstrated the greater importance of the increase of ANP level with sacubitril/valsartan and the increase of cGMP.67 In women, there is the possibility that the decrease of oestrogen-dependent stimulation of natriuretic peptides after menopause cause a further reduction of the NO/cGMP/PKG pathway, whose activation can be highly beneficial in women compared with men, and this benefit can be higher with the same serum and urinary levels of cGMP (therefore the same level of urinary cGMP in both sexes in PARAGON-HF does not exclude this hypothesis). Finally, the inhibition by ARNI of neprilysin has multiple benefits that we still only partially know because neprilysin inactivates multiple biological substances with potentially different repercussions in each sex.
Taking these considerations together, the use of ARNI in HFpEF in specific populations of women, such as those who are older and obese, can be considered to improve QoL by reducing HHF.
New Molecules with Impact on Quality of Life in HFrEF
Vericiguat
Vericiguat is a new drug that increases cGMP with a double mechanism, one side directly stimulates the soluble guanylate cyclase (sGC) through a binding site independent of NO and the other sensitises sGC to endogenous NO by stabilising the NO-sGC binding site. The final effect is an important boost of the NO/cGMP/PKG pathway. We previously mentioned this pathway because it is partially activated also by ARNI and SGLT2i. However, vericiguat specifically activates this pathway that has beneficial effects both in diastolic and in systolic ventricular dysfunction and in patients with HF in whom the oxidative stress leads to a reduction of NO and to cGMP deficiency. VICTORIA trial – dedicated to vericiguat in HFrEF – enrolled and randomised 5,050 patients (key inclusion criteria in Table 2).68,69
Vericiguat was started at a dosage of 2.5 mg/day at randomisation and biweekly uptitrated in a blinded fashion to reach the target dose of 10 mg per day (if tolerated based on mean SBP and clinical symptoms). After 10.8 months of mean follow-up, vericiguat significantly reduced the primary endpoint: a composite of CV death and first HHF [HR 0.90 [0.82–0.98]; p=0.02, RRR 10%). However, the benefit was significant only for the first HHF (HR 0.90; 95% CI [0.81–1.00]; p=0.048, RRR 10%] but not for CV death (HR 0.93; 95% CI [0.81–1.06]; p=0.269). The secondary endpoints confirmed the benefit on HHF, indeed total (first and recurrent) HHF were significantly reduced (HR 0.91; 95% CI [0.84–0.99]; p=0.023, RRR 9%) with no effect on all-cause death (HR 0.95; 95% CI [0.84–1.07] p=0.38).
Although at a first and superficial glance the results achieved in VICTORIA do not seem to be remarkable, the understanding of the characteristics of the enrolled population explains their important impact on HFrEF. Indeed, the population enrolled in VICTORIA had very severe HF when compared to all the recent RCTs dedicated to HFrEF (Table 2), which included 23.9% of women, with higher NYHA III class 39.7%, mean LVEF 28.9%, very high levels of median NT-pro-BNP = 2,816 pg/ml (double DAPA-HF and significantly higher than the other RCTs), ~84% had an HHF within 6 months, with the remaining ~16% on IV diuretics managed as outpatients, a compromised renal function superimposable to that of EMPEROR-Reduced (mean eGFR 61.5 ml/min/1.7m2, 52% had eGFR <60 ml/min/1.73 m2), and 46.9% with T2D. The HFrEF OMT was comparable to that of DAPA-HF and EMPEROR-Reduced and included 14.5% of patients on ARNI, 59.7% having triple therapy (ACE-I/ARB/ARNI, β-blocker, MRA), 27.8% with an ICD and 14.7% with a CRT (Table 2).
The HFrEF severity of VICTORIA’s population is well described by the annualised event rate in the comparator group. indeed the annual rate of CV death was two times higher than that of PARADIGM-HF, DAPA-HF, and EMPEROR-Reduced and the annual rate of first HHF rate was three times higher than that of PARADIGM-HF and DAPA-HF and twice as high as that of EMPEROR-Reduced (Table 2). Add to this, the rate of all-cause death in the comparator group was 21.2% in 10.8 months similar to that of PARADIGM-HF (19.8%) with the noticeable difference that this rate was achieved in 27 months. These numbers are close to the event rate that we observe in clinical registers and in our clinical practice in HFrEF patients and explain what type of patients were enrolled in this trial. The benefit of VICTORIA on primary endpoint in terms of absolute RR was comparable to that of DAPA-HF and was mostly due to the benefit on HHF.70 Furthermore, this benefit was achieved in a very short mean follow-up and it is likely that if the mean follow-up was longer, the benefit would have been even greater.
There were no major safety issues with vericiguat. In particular, there were no significant differences in terms of syncope and hypotension, while there was an increase in the rate of anaemia (7.6% versus 5.7% of placebo). Particularly, if the SBP was ≥90 and <100 mmHg the dosage was maintained, whereas in the case of SBP <90 mmHg the vericiguat dose was reduced. Therefore, this drug has no negative impact on hypotensive patients.
In summary, this drug is highly beneficial in patients with recent HHF (<6 months) who have severe HF (as defined by the characteristics of the enrolled population) and present evident (clinical and laboratory) signs of congestion and can be considered in hypotensive patients not able to tolerate ACEi/ARB/ARNI, as already discussed for SGLT2is. Furthermore, the selective activation and boosting of the NO/cGMP/PKG pathway combines the myocardial benefits (inhibition of hypertrophy, fibrosis, inflammation and increase of coronary blood flow) that cause an improvement of diastolic function, ventricular-arterial coupling and an anti-remodelling effect with the vascular benefits (inhibition of inflammation, vasodilative properties and positive effect on vasal remodelling) that may have positive consequences, especially in women. Recently our group provided a proof-of-concept study that demonstrated a compensatory active role of arteries in HF across the LVEF spectrum. New drugs, such as vericiguat specifically act on arterial function and can be more beneficial in specific populations with a more compromised arterial function – such as older women with HF and HFpEF patients.71
Omecamtiv Mecarbil
Omecamtiv mecarbil (OM), is a selective cardiac myosin activator that increases cardiac contractility by specifically binding to myosin, stabilising the pre-powerstroke state prior to onset of cardiac contraction with the effect of increasing the number of myosin heads that can bind to the actin filament and undergo a powerstroke once the cardiac cycle starts. Its action can be described as ‘more hands pulling on the rope’. Moreover, OM decreases the turnover of adenosine triphosphate in the absence of an interaction with the actin filament, potentially increasing the overall energetic efficiency of the system by diminishing adenosine triphosphate use not associated with mechanical work.72 This peculiar mechanism does not increase myocyte calcium and does not increase myocardial consumption of oxygen. This clearly differentiates this drug from other inotropes (such as β-adrenergic receptor agonists, phosphodiesterase inhibitors and levosimendan). OM has the final effect to increase the stroke volume, the left ventricle ejection time and systole duration, also reducing heart rate. OM has shown multiple benefits in HFrEF patients in Phase II trials, which has led to the development of a large Phase III RCT.73,74
GALACTIC-HF – dedicated to OM in HFrEF – enrolled and randomised 8,442 patients with a severe HF when compared to other recent RCTs (Supplementary Material Table 1 and Table 2): 21.3% were women, NYHA III represented 43.8% of patients, median LVEF was particularly low 26.6%, median NT-proBNP was similar to that of EMPEROR-Reduced 1,971 pg/ml, 54.6% had a recent HHF (within 6 months), interestingly 25.2% had AHF, median eGFR:60.3 ml/min/1.73 m2, T2D patients were 40.1%. Patients were very well treated as for other RCTs (Table 2).76 After 21.8 months of mean follow-up, OM – titrated from 25 to 50 mg twice daily to reach a specific plasmatic concentration – significantly reduced the primary endpoint: a composite of CV death and first HF event (defined by an HHF or emergency department access for HF or urgent visit for WHF), HR 0.92 95% CI [0.86–0.99]; p=0.025; RRR 9%. However, when considered alone none of the individual components of primary endpoint were significantly reduced (CV death: HR 1.01 95% CI [0.92–1.11]; p=0.86; first HF event: HR 0.93 95% CI [0.86–1.00], p=0.06) as well as the secondary endpoint all-cause death (HR 1.00 95% CI [0.92–1.09]; p=not significant). Beyond the major outcome, QoL measured by KCCQ (another secondary endpoint) was significantly improved. As we previously mentioned for VICTORIA, the severity of the population enrolled in GALACTIC-HF is well described by the annualised event rate of CV death in the comparator group – 10% higher than in other recent HFrEF RCTs (only inferior to that of VICTORIA) and of first HF event rate 15.2% (the same as EMPEROR-Reduced; Supplementary Material Table 1).
Pre-specified group analysis showed that the benefit for primary endpoint was particularly significant for people with a median LVEF <28.0% (HR 0.84; 95% CI [0.77–0.92], p=0.03 for interaction:).76 Regarding safety, OM did not significantly increase ventricular tachyarrhythmias, torsades de pointes or QT prolongation, MI or any serious adverse events. Further, post-hoc analysis provided generator hypotheses of greater benefit in more advanced HF populations (LVEF <28% plus one of the following: HHF within 3 months, NYHA III/IV, NT-pro-BNP >2,000 pg/ml, SBP 2.85 mmol/l.) that need to be confirmed in future studies. On the other hand, COSMIC-HF has also demonstrated that OM significantly increases stroke volume, systolic ejection time, reduces ventricular volumes, NT-pro-BNP levels and heart rate and these actions are demonstrated as also significant for the right ventricle.77
In summary, this is the first inotrope in the history of cardiology that does not increase CV death and all-cause death, does not have any adverse events and that is active (significantly reducing the primary endpoint) on a population of advanced HF acting on both ventricles without any reduction of SBP. Further studies will clarify the benefits of OM, but its introduction to HF therapies can be an added value in women with severe HF who are unable to tolerate ACEi/ARB/ARNI or high dosage of β-blockers because of hypotension. In this population of advanced HFrEF, a combination of drugs without any effect on SBP, such as SGLT2i, MRA, vericiguat and OM should be considered, not only improving outcomes and QoL but also generating amelioration myocardial and vascular function that makes it possible to start and titrate ACEi/ARB/ARNI.
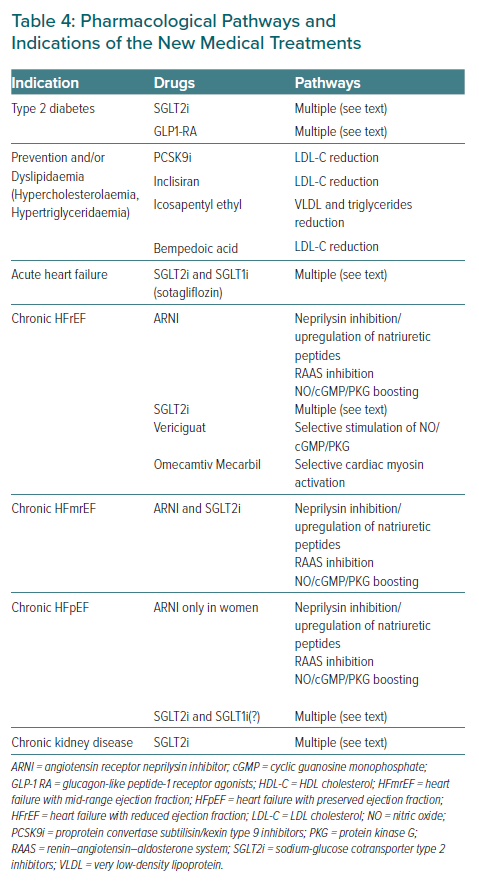
Table 4 summarises the indications of the new medical treatments in women from prevention to heart failure management.
Conclusion
Cardiovascular risk remains high for women with diabetes and is particularly underestimated – because the SCORE chart does not take into account specific women’s risk factors, such as premature menopause or peripartum complications, and this contributes to inadequate prevention strategies in this population. The suboptimal management of women with diabetes leads them to progress to overt HF. New therapeutic approaches offered by new drugs have shown evidence of multiple benefits on CV morbidity and mortality in women. A better implementation of new pharmacological treatments both in CV prevention and in HF is an unmet need for women with diabetes and requires a greater commitment from the medical community to improve women’s health.
A specific focus on benefits of new drugs that potentially have greater effect in women than in men – as may be the case for ARNI as shown in PARAGON-HF – and on specific pathways, potentially more important in women than in men – as is the case of NO/cGMP/PKG – can lead us to develop a tailored-approach using ‘precision medicine’.
Click here to view Supplementary Material.