Direct oral anticoagulants (DOACs) incur a higher risk of gastrointestinal bleeding, but an overall lower bleeding risk, and significantly lower risk of severe and intracerebral bleeding compared with warfarin. All of the current DOACs, except for dabigatran target factor Xa, inhibit both thrombus formation and haemostasis. Factor XI may be involved in thrombus formation, but only to a lesser extent involved in haemostasis. Thus, the inhibition of factor XI may regulate pathological thrombus formation without the risk of increased bleeding.
Several novel factor XIa (FXIa) inhibitors are under investigation for thromboprophylaxis in high-bleeding risk populations. Asundexian is a small molecule that selectively inhibits FXIa. Other FXIa inhibitors under development include an antisense oligonucleotide (ISIS-416858) and antibodies against FXIa (MAA868 and BAY1213790). In the AXIOMATICTKR Phase II trial, the oral low-molecular-weight FXIa inhibitor, milvexian, decreased the occurrence of venous thromboembolism in a dosedependent manner after total knee replacement surgery compared with enoxaparin, a low-molecular-weight heparin.1,2 However, the bleeding risk for milvexian was similar to the bleeding risk for enoxaparin.
PACIFIC-AF is a multicentre, randomised, double-blind, Phase II clinical trial to investigate the efficacy of the FXIa inhibitor, asundexian, in patients with AF and increased bleeding risk.3 Patients were randomised into groups receiving asundexian 20 mg or 50 mg once daily, or apixaban 5 mg or 2.5 mg twice daily, according to the Food and Drug Administration labelling. The primary endpoint was the composite of major bleeding or clinically significant non-major bleeding.
Asundexian inhibited FXIa activity by 81% and 90% of the baseline at trough and peak concentrations in the 20 mg group, and 92% and 94% in the 50 mg group, respectively. The relative risk reduction of the primary endpoint compared with apixaban was 50% for 20 mg asundexian, 84% for 50 mg asundexian and 67% for overall asundexian. In this trial, 29% of the patients (216/755) had chronic kidney disease, defined as a creatinine clearance of 30–50 ml/min, a status with higher risk for both bleeding and thrombosis.
PACIFIC-AF is the first human trial to show that an FXIa inhibitor reduced bleeding compared with the standard treatment. However, PACIFIC-AF was designed as a Phase II study to determine dosage, and the study was not powered to determine differences in thrombosis rates between groups. Furthermore, the number of bleeding events was only half of the anticipated number (10 instead of 20 events), and no major bleeding events were observed. The authors highlight the strong correlation between minor and major bleeding in other anticoagulation trials.4 Patients lacking FXI are not more prone to intracranial or gastrointestinal haemorrhage than the general population. Thus, FXIa inhibitors may not only be associated with fewer bleeding episodes, but also with less lifethreatening bleeding events.
Similarly, the results of the AZALEA-TIMI 71 trial were recently presented.5 This Phase II trial indicated that abelacimab, a highly selective fully human monoclonal antibody to FXI, was associated with a lower risk of bleeding compared with rivaroxaban 20 mg daily among patients with AF and a high CHA2DS2-VASc score. The trial was stopped prematurely by its data monitoring committee due to an overwhelming reduction in bleeding. However, both PACIFIC-AF and AZALEA-TIMI 71 trials were limited by the small sample size, short follow-up time and insufficient assessment of the efficacy of anticoagulation therapy.
A large-scale Phase III clinical trial called OCEANIC-AF has been initiated in >40 countries, aiming to enrol a total of 18,000 patients. The efficacy and safety will be compared between asundexian and apixaban.6 The clinical use of anticoagulants for patients with chronic kidney disease could change based on the findings of the OCEANIC-AF trial. However, the OCEANIC-AF study was recently halted due to the inferior efficacy of asundexian versus the apixaban control arm.6 Available safety data are consistent with previously reported safety profiles of asundexian. This trial underlines the necessity to conduct large, randomised control trials to assess both safety (bleeding) and efficacy (thromboembolism prevention).
An independent data monitoring committee recommended that another Phase III trial, OCEANIC-STROKE, in patients who had already suffered a stroke, continue. Detailed data will be analysed in the future, but based on what is currently known, FXIa inhibitors (asundexian) have a significantly lower bleeding risk, but weaker anticoagulant actions than factor Xa inhibitors (apixaban). In the treatment of atherosclerotic cardiovascular disease, the addition of a small amount of the anticoagulant, rivaroxaban, to antiplatelet drugs, so-called dual pathway inhibition, increases the risk of bleeding, but has been shown to be effective in suppressing ischaemic events. Based on these facts, FXIa inhibitors, such as asundexian, may be useful in the future in clinical practice for atherosclerotic cardiovascular diseases.
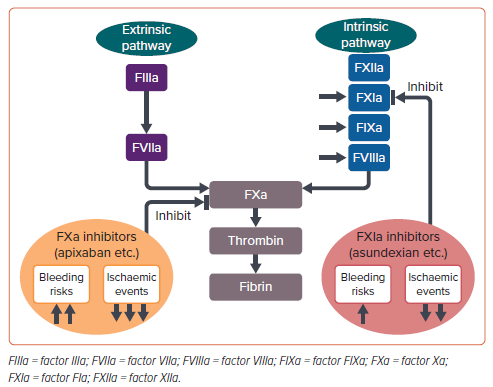
Why did OCEANIC-AF fail? Asundexian has been shown to almost completely inhibit FXIa in vivo by >90% at a dose of 20 mg. Therefore, it is unlikely that the dose was too low. Rather, it would be reasonable to interpret that inhibition of only the intrinsic coagulation pathway results in weaker suppression of the final coagulation activity than inhibition of both the intrinsic and extrinsic pathways. FXIa inhibitors are marginally less effective, but significantly safer than factor Xa inhibitors (Figure 1). Therefore, even in AF, if appropriate patients are selected, there is a possibility that the benefits of FXIa inhibitors, which are much safer, may outweigh their limitations despite being slightly less effective.
Then what is the future position of FXIa inhibitors in AF? Low-dose edoxaban 15 mg once daily was tested in very elderly Japanese AF patients (aged ≥80 years) who were not appropriate candidates for the standard DOAC regimen.7 The eligibility criteria included low creatinine clearance (15–30 ml/min), a history of bleeding from a critical area or organ or gastrointestinal bleeding, low bodyweight (≤45 kg), continuous use of nonsteroidal anti-inflammatory drugs or current use of an antiplatelet drug. The results demonstrated that edoxaban 15 mg once daily prevented stroke or systemic embolism better than placebo and did not result in a significantly higher incidence of major bleeding compared with placebo.7 Thus, FXIa inhibitors could be a therapeutic option for patients who cannot tolerate standard DOAC treatment.
Advances in the development and potential applications of FXIa inhibitors in select high-bleeding risk patients are expected in the future.










