Chronic kidney disease (CKD) is a major global public health problem, and is defined as a decrease in estimated glomerular filtration rate (eGFR) below 60 ml/min/1.73 m2 for >3 months or pathological abnormalities of kidney structure or function with preserved GFR.1–3 It is estimated that 9.1% of the global population has CKD, with this percentage almost quadrupling in patients >70 years of age.1,4,5
Patients with CKD, those with end-stage renal disease (ESRD) on haemodialysis and kidney transplant recipients have an increased risk of developing cardiovascular disease (CVD), which includes cardiomyopathy, coronary disease, arrhythmia and valvular heart disease (VHD).5–7 More than half of the ESRD patients on dialysis have concomitant CVD.8 This coexistent disease is termed cardiorenal syndrome.6,9 Due to the complexity of simultaneous cardiac and renal disease, it is difficult to determine which is primary and secondary; however, the mixed disease significantly increases morbidity, mortality and healthcare costs.10
Mitral and Aortic Valve Disease
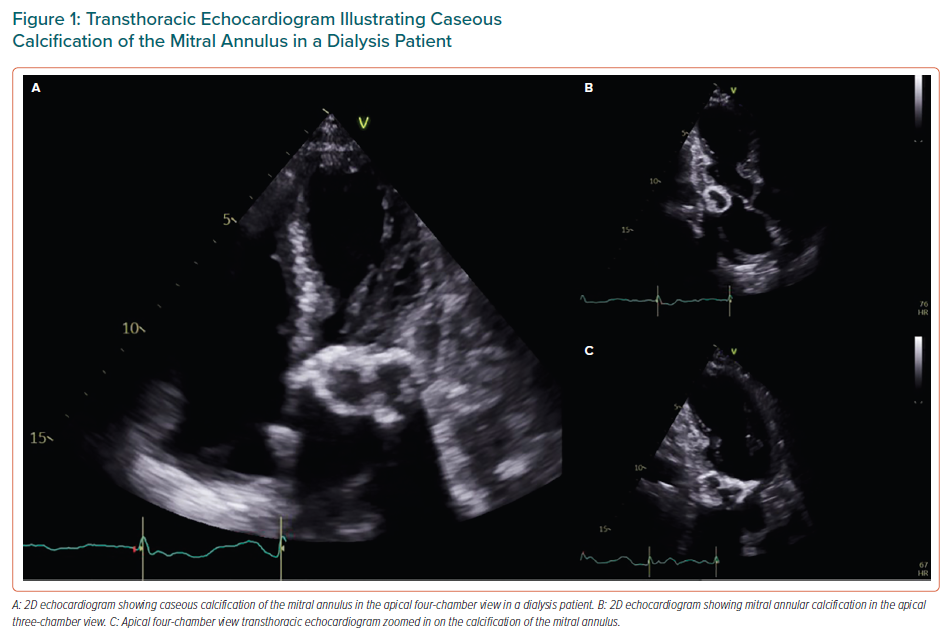
VHD is prevalent in patients with CKD (early stages of the disease) and ESRD.8 Aortic and mitral valves are the most frequently affected, leading to aortic valve (AV) calcification or mitral annular calcification (MAC), which, in turn, causes either valve stenosis or regurgitation.6,11 AV disease constitutes the most common VHD in CKD patients and in ESRD patients on haemodialysis.12–14 Mitral valve (MV) disease comes second, with the exception of a few regional variations in which MV disease is the leading VHD in patients with renal insufficiency.6,12–15 Caseous calcification of the mitral annulus and MAC-related calcified amorphous tumour are two variants of MAC that occur particularly in patients with ESRD (Figure 1).16 Moreover, it is observed that the greater the impairment in renal function, and therefore the lower GFR, the greater the prevalence of VHD, with most affected patients being at a later stage of CKD (Stage 5 or GFR <15 ml/min/1.73 m2) rather than in an earlier stage (Stage 1 and 2, or GFR >60 ml/min/1.73 m2).8,13,17–19
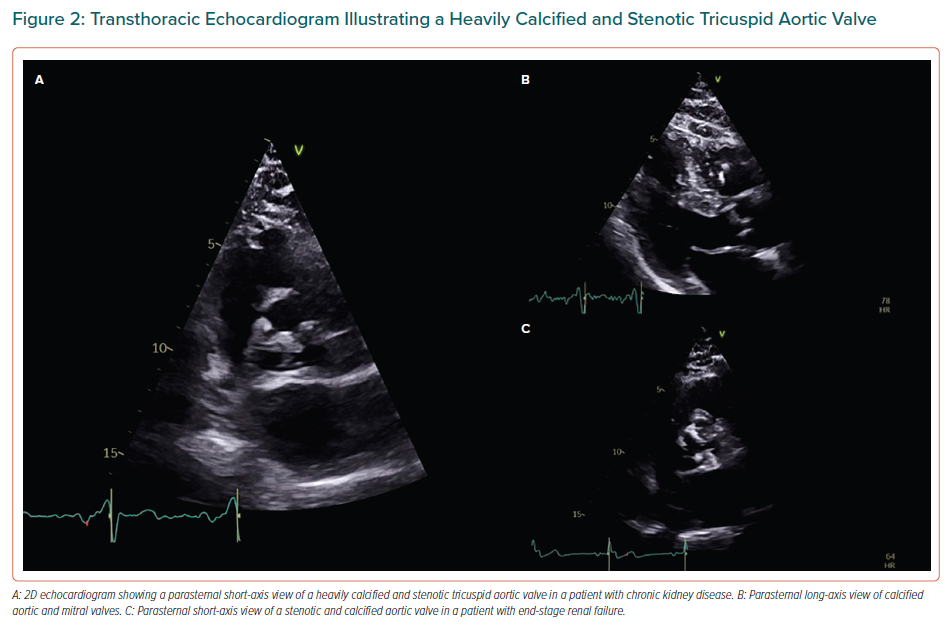
It has been established that the rate of valve calcification is accelerated in ESRD patients compared with the general population, and it can be up to 10-fold quicker in ESRD patients compared with patients with milder stages of CKD and not undergoing haemodialysis.8,16,20–23 More specifically, it has been reported that AV calcification progresses to severe aortic stenosis (AS) one to two decades earlier in CKD/ESRD patients than in the general population (Figure 2).8,11,18,24 Published data on this issue has demonstrated that the change in AV area is more than double (-0.19 cm2/year) compared with patients without CKD (-0.7 cm2/year).13 A similar pattern to native valves is observed in bioprosthetic valves, where the structural deterioration occurs more quickly in CKD patients than in the general population.11
Valve Regurgitation
According to most published work, the structures of left-sided valves that are commonly affected by calcification and thickening are the mitral annulus and the aortic cusps, causing valve regurgitation, valve stenosis or both.25 Aortic regurgitation is usually organic and is caused by calcification of the aortic cusps. Mitral regurgitation (MR) may be either organic, due to MAC, or functional, due to the intravascular volume overload that is found in this population (e.g. hypertension, anaemia).13 The latter is also responsible for tricuspid regurgitation (TR), which, in most cases, is functional.13
Valve Stenosis
Calcific AS is the most prevalent VHD among patients with ESRD.8,13,26 Mitral stenosis in this population typically includes severe MAC (mostly of the posterior mitral annulus) and thickening of the base of the mitral leaflets, whereas the leaflet tips and commissures are usually less affected.27 For this reason, the incidental finding of MAC in the echocardiogram of a younger adult should arouse suspicion and should be investigated further.28 In contrast to CKD mitral stenosis, it is worth mentioning that in the case of radiation-induced mitral stenosis there is also severe MAC, but mostly in the anterior annulus, as well as calcification of the aortomitral curtain.28 In addition, thickening can be observed at the base or the mid-body of the anterior mitral leaflet.28 Finally, in the case of rheumatic mitral stenosis, calcification and thickening of the subvalvular apparatus, commissural fusion and thickening of the tips of the leaflets are observed.28 However, diagnosis of the aetiology of mitral stenosis is not predominantly based on the above imaging characteristics, but rather on the clinical history. In some cases, mitral stenosis can be also mixed.
Epidemiology
Recent published results show that the prevalence of MAC ranges from 5% to 60% in patients with CKD.8,11,18 In dialysis patients, the reported prevalence ranges from 25% to 59%, especially in those undergoing haemodialysis for >3 years.8,11,12,16,28 MAC occurs approximately four times more often in patients with renal insufficiency compared to the general population, in which prevalence ranges from 8% to 15% depending on age, with the greatest prevalence seen at an advanced age.29 MAC is more commonly seen in ESRD than CKD.8,11,12,16,18
Conversely, the prevalence of AV calcification ranges from 23% to 85% in CKD patients, and from 28% to 76.5% in dialysis patients.8,11,12,13,16,18 Consequently, we can infer that AV calcification is also markedly prevalent in these patient populations, occurring approximately three times more often than in the general population, in which the prevalence of calcific aortic sclerosis increases with age and occurs in approximately 25% of individuals aged >65 years.13 The prevalence of AV calcification is higher in ESRD than CKD patients.8,11,12,16 In dialysis patients, the prevalence of severe AS ranges from 4% to 13%, with more than half of these patients having low-flow, low-gradient severe AS.11–13 Severe AS is also highly prevalent in ESRD patients compared with the general population, in which the prevalence of severe AS is approximately 3% in individuals aged >75 years, with prevalence increasing with age.13,23,24,26
The prevalence of CKD in patients with severe AS undergoing AV replacement (AVR), either transcatheter (TAVR) or surgical (SAVR), is approximately 50–75%.30–32 Most of these patients have moderate to severe CKD rather than mild to moderate CKD.30–32 Thus, impaired renal function constitutes a common characteristic in patients undergoing AVR. One possible explanation is that longstanding AS impairs forward blood flow from the heart, which, in turn, causes hypoperfusion of the kidneys, leading to CKD.26,33 For this reason, renal dysfunction may be the ‘first symptom’ observed in patients with severe AS.34,35 The prevalence of preoperative advanced CKD, namely GFR <30 ml/min/1.73 m2, is 11.5% in patients with severe MR receiving a MitraClip.36 In addition, CKD is as prevalent in VHD patients as VHD is in CKD patients, with the prevalence of CKD ranging between 25% and 75% in patients with significant VHD.20,23,37–39
Pathophysiology
Several theories have been proposed attempting to explain the mechanisms of VHD in CKD patients. These mechanisms are divided into three main categories, namely traditional and non-traditional risk factors for CVD and CKD-related risk factors. Traditional CVD risk factors include advanced age, diabetes, smoking history, hypertension, lipid and lipoprotein metabolism disorders (oxidised LDL, lipoprotein(a)) and obesity.5,6,11,16,18 As such, it is clear that valve and vascular calcification share the same risk factors. Non-traditional risk factors include increases in serum parathyroid hormone concentrations, hyperphosphataemia, hypocalcaemia and vitamin D deficiency.11,18 For this reason, the use of either vitamin K antagonists for non-valvular AF in ESRD patients or excessive vitamin D supplementation in CKD patients raises concerns.11,18 CKD-related risk factors include shear stress-related valvular endothelial damage, anaemia, uraemic toxins and mineral bone disorders (osteoprotegerin, the RANK/receptor activator of nuclear factor-κB ligand [RANKL] axis, fibroblast growth factor 23/klotho), which constitute one of the most important mechanisms causing valve calcification in the early stages of CKD.11,13,16,18,40 The mechanism accelerating VHD in CKD patients appears to be a combination of traditional and non-traditional CVD risk factors and CKD-related risk factors.
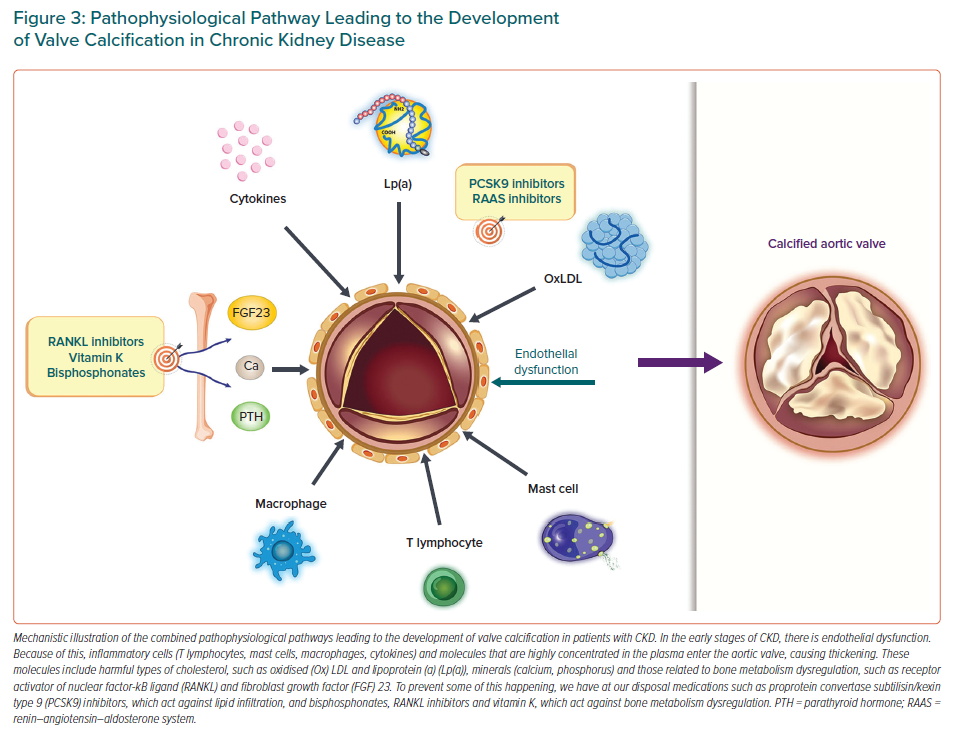
Chronic systemic and local inflammation (T lymphocytes, mast cells, macrophages, cytokines) with increased oxidative stress in the calcified and pericalcific regions of the valves constitute additional mechanistic pathways.5,6,11,16 Other possible pathological mechanisms that have been proposed include malnutrition, cachexia, hyperuricaemia and increased sclerostin concentrations.15,18,41,42 The presence of systemic shunting due to dialysis arteriovenous fistulas, which create a volume overload state on the right heart chambers, in turn causing right ventricular dilatation and dysfunction, may also contribute to VHD.13 This leads first to worsening of TR, followed by exacerbation of MR.13 The fact that different pathophysiological mechanisms causing VHD in CKD patients have been put forward highlights the complexity of the calcification process and may suggest that different mediators may be required at different stages of the process (Figure 3).43
Clinical Presentation
Prior to the typical manifestation of symptomatic VHD, CKD patients commonly have a long asymptomatic period, which may be preceded by trivial symptoms, which are not always obvious due to the inactivity of CKD patients.13 During the symptomatic phase, patients may manifest typical symptoms such as dyspnoea, but also atypical symptoms such as fatigue and muscle weakness, which may be mistakenly attributed to other coexisting conditions such as anaemia and frailty.13 More specifically, CKD patients with AS may manifest one of the three standard symptoms, namely angina, heart failure (e.g. pulmonary oedema) and syncope/sudden cardiac death.44 CKD patients with AR may develop dyspnoea, palpitations due to frequent and premature left ventricular (LV) beats and peripheral oedema.44 When CKD patients experience MR, they may develop dyspnoea, fatigue, AF and pulmonary oedema.44 Finally, those with MV stenosis may develop AF, thromboembolic stroke and symptoms and signs due to right ventricular failure, such as fatigue and peripheral oedemahepatosplenomegaly.44 Auscultation will reveal the respective standard murmurs in each of these four categories of VHD patients.13,44 Due to the long asymptomatic period, echocardiography plays a major role in the diagnosis of VHD and should always be readily available so that VHD can be promptly diagnosed.13
Diagnosis
Echocardiography plays a leading role in the diagnosis of VHD in CKD/ESRD patients and constitutes the gold standard for the assessment of valve morphology and the quantification of valve stenosis and/or regurgitation.8,13 The updated 2017 Kidney Disease: Improving Global Outcomes (KDIGO) CKD–mineral bone disorder recommendations suggest that a transthoracic echocardiogram is within the framework of the screening process to detect the presence or absence of VHD in patients with CKD Stage 3a to 5 (GFR <59 ml/min/1.73 m2).13 In the case of valve regurgitation, it is recommended that the echocardiogram is performed on a post-dialysis day, when the patient has their ‘dry weight’, and with blood pressure under control.3
During a routine echocardiogram, apart from assessing the heart valves, it is also important to assess the LV geometric pattern, given that more than half of all ESRD patients have hypertrophy, with concentric hypertrophy the predominant pattern.12,45 In addition, measurement of the LV ejection fraction (LVEF), assessment of LV diastolic function, given that most patients have an impaired relaxation pattern, and measurement of LV global longitudinal strain may help risk stratify patients with renal insufficiency.12,16
Echocardiography plays a central role in the decision-making process for patients with severe AS and CKD who may benefit from valve replacement. More specifically, prognosis and post-surgical outcome can be predicted using simple and reproducible echocardiographic markers, including relative wall thickness, LV mass index, LVEF, estimated LV filling pressure (E/e¢ ratio), left atrial volume index, pulmonary artery systolic pressure (PASP) and mean transaortic pressure gradient.40,46 In addition, echocardiography is important for the follow-up and surveillance of patients with VHD. As per the VHD management guidelines, patients must have a follow-up within 3–6 months in the case of severe VHD, within 6–12 months in the case of moderate VHD and within 12 months in the case of mild VHD.11,13 A yearly follow-up of the prosthesis using echocardiography is indicated in patients who have undergone bioprosthetic valve replacement.11
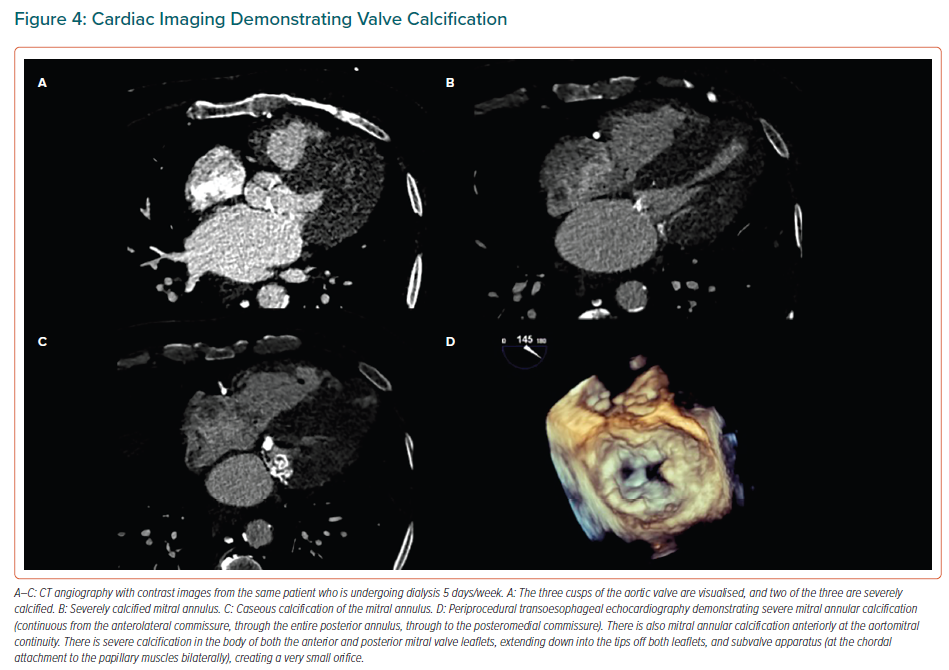
Despite progress in techniques such as cardiac CT and MRI in recent years, these technologies do not have a major role in the diagnosis of VHD in patients with impaired renal function compared with echocardiography, which, importantly, may be due to the risk of developing contrast-induced nephropathy.16 More specifically, cardiac CT is supplementary to echocardiography for the quantification of valve calcification, measuring the calcium of the AV and the mitral annulus (Figure 4).13,47,48 Cardiac CT provides additional information about the exact degree of AS in the case of both low-flow, low-gradient severe AS with reduced ejection fraction and low-flow, low-gradient AS with preserved ejection fraction.13,47,48
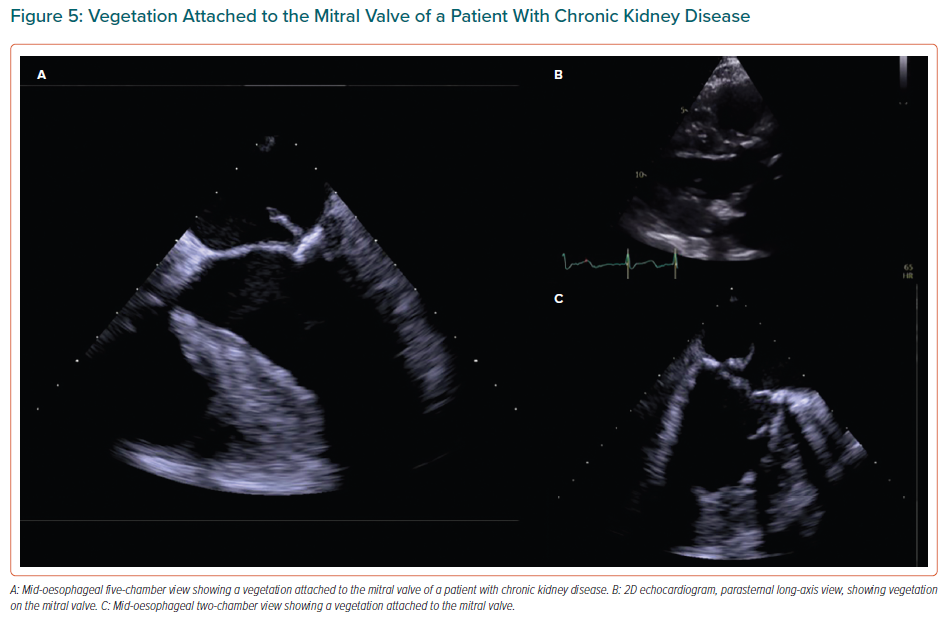
In addition, cardiac CT is performed in CKD patients prior to transcatheter valve replacement in order to assess the morphology and size of the aorta and aortic annulus.13,48 Before valve replacement, it is recommended that coronary angiography is performed to assess coronary artery disease.8 In case of low-flow, low-gradient severe AS with reduced ejection fraction, a low-dose dobutamine stress echocardiogram may also be performed for differential diagnosis between truly severe and pseudo-severe AS and in order to determine whether there is contractile reserve.13,47 Dialysis patients have an increased risk of developing infective endocarditis and, if suspected, a transoesophageal echocardiogram should be performed (Figure 5).8 A chest X-ray has a low sensitivity to diagnose VHD in ESRD patients.48
Prognosis
CKD constitutes one of the leading causes of global mortality, and is ranked 12th among the global list of deaths in 2017.1 It is also known that CKD patients have significantly greater all-cause and cardiovascular morbidity and mortality than those with normal renal function, with CVD usually the cause of death, as opposed to renal impairment.11,16,22,49 In addition, the greater the attenuation in GFR, the higher the mortality rate, with ERSD patients on haemodialysis having a ninefold higher mortality rate than the general population.11,16,41 It is evident that CKD patients with concomitant severe AS who are treated conservatively have a poor prognosis, with significantly higher mortality rates than those treated with valve replacement.22,30,38
Both SAVR and TAVR have been recognised as superior to medical treatment in terms of mortality in patients with impaired renal function.22 This is the case even in dialysis and advanced CKD patients (GFR <30 ml/min/1.73 m2), who have high mortality rates after any kind of cardiac intervention.39,50–54 More specifically, dialysis patients have a 30-day mortality rate of up to 21% following SAVR and a 1-year mortality rate of between 34% and 53% following SAVR.23,32,52,55 These high mortality outcomes have led to the use of TAVR. In ESRD patients undergoing haemodialysis, TAVR is associated with a 30-day mortality rate of up to 8.3%, a 1-year mortality rate of up to 36.8% and a 2-year mortality rate of up to 50%, with sepsis being the most common cause of death.52,55–57
There are conflicting views regarding improvements in renal function following valve replacement in the literature, with some authors reporting an improvement33,34,58 and others reporting a decline.59 Undoubtedly, those who manifest postoperative acute kidney injury (AKI) have significantly elevated mortality risks.13,22,52,56,60–62 Regarding mortality rates of other cardiac interventions, the 30-day mortality rate of CKD patients undergoing balloon aortic valvotomy is up to 10.2%.63 Similar to AVR, the mortality rates after MitraClip implantation increase significantly, up to 53%, when GFR is <30 ml/min/1.73 m2.36,64,65 The effect of postoperative AKI on mortality risk after MV replacement is similar to that after AV replacement.65 Importantly, as many as 30% of CKD patients with severe VHD on the waiting list for surgery will die before receiving treatment, which shows how cardiologically unstable they are.38
Treatment
There are currently no specific guidelines for the management of VHD in non-severe CKD patients (GFR ≥30 ml/min/1.73 m2). Consequently, the existing 2017 European Society of Cardiology (ECS)/European Association for Cardio-Thoracic Surgery guidelines addressing patients with normal kidney function are used.8 As such, for the AV, SAVR is recommended in severe AS patients with low perioperative risk (AV area ≤1 cm2 or ≤0.65 cm2/m2, mean gradient ≥40 mmHg or peak velocity ≥4 m/s) when they manifest symptoms (indication class 1, level b) or when they present with hypotension during exercise testing (class 1, level c), or when LVEF is <50% (class 1, level c) or when one of the following is present: peak velocity across the AV >5.5 m/s, severe valve calcification with a peak velocity progression of ≥0.3m/s per year, markedly elevated neurohormone concentrations and PASP >60 mmHg (class 2a, level c). 8,37,47,54 Transcatheter AV implantation is recommended in elderly patients (age >75 years) with severe symptomatic AS when they are not suitable for SAVR due to prohibitive, high or intermediate surgical risk (Society of Thoracic Surgeons/EuroSCORE II ≥4%, logistic EuroSCORE I ≥10%; class 1, level b).47,49,50,56,66 Balloon aortic valvotomy may be considered as a link to either SAVR or TAVR in unstable patients or in patients with symptomatic severe AS who must immediately undergo major non-cardiac surgery (class 2b, level c).47,63
As for the MV, surgery or repair whenever possible is recommended in symptomatic patients with severe primary MR who have an LVEF >30% (class 1, level b).47 Surgery is also indicated in asymptomatic patients with severe primary MR when there is concomitant LV dysfunction, defined as LVEF ≤60% or LV end systolic diameter ≥45 mm (class 1, level b).47 MV surgery should also be considered in asymptomatic patients with preserved LV function when there is newly diagnosed AF secondary to MR, or there is pulmonary hypertension defined as PASP >50 mmHg (class 2a, level b).47 MV repair is preferred over MV replacement whenever possible.47 Finally, percutaneous MV repair (transcatheter MV repair) with the MitraClip device may be considered in patients who are characterised as inoperable or who have high surgical risk, because they meet all the required echocardiographic criteria (class 2b, level c).47
Either percutaneous mitral commissurotomy or MV surgery is indicated in symptomatic patients with clinically significant mitral stenosis, which is defined as a valve area <1.5 cm2 (class 1, level b or c).47 The final therapeutic option is based on the clinical and anatomical characteristics of these patients.47 Surgical or percutaneous treatments are not recommended in patients with significant mitral stenosis and who are asymptomatic, as confirmed by stress testing, unless there is an increased risk of systemic embolism or haemodynamic decompensation (class 2a, level c).47 For CKD patients, commissurotomy is often not feasible due to extensive valve and subvalve calcification. Medication is not effective in the management of VHD because it does not stop or slow down the progression of calcification in patients with and without CKD.11,48,49 Studies have also shown the beneficial effect of the administration of levosimendan on the incidence of AKI in patients who have preoperative CKD and who are haemodynamically unstable postoperatively.61
In circumstances where the recommendation is valve replacement, there are two prosthetic options: bioprosthesis and mechanical valves.47 In patients with renal impairment, the selection of valve type is determined by the patient’s age, comorbidities, life expectancy, surgical risk, a patient’s personal preference and the probability of accelerating structural valve deterioration due to their main disease, which is the same as for patients without renal impairment.11,47 Generally speaking, bioprosthetic valves are preferred for older patients (>60 years old) as well as for people who do not desire to use anticoagulation in the long term or who are at a high surgical risk.11,47 Conversely, bioprosthetic valves are more prone to rapid structural deterioration in dialysis-dependent patients.47 Therefore, bioprosthetic valves are usually offered to advanced CKD patients because they have a short life expectancy, a low probability of reoperation and a problematic use of vitamin K antagonists.11
Tricuspid Regurgitation
Compared with the bibliography focusing on left-sided valves and CKD, the bibliography focusing on TR is much less. TR is prevalent in CKD and ESRD and due to pulmonary hypertension, which, in turn, is due to high LV filling pressures.67 In most cases TR is functional and thus potentially reversible.13 Treatment involves strict fluid balance and diuretics. Prognosis is poor, especially with the onset of heart failure.68 According to the current European guidelines, TV surgery is indicated in patients suffering from severe primary or secondary TR and who are also undergoing left-sided valve surgery (class 1, level c).47 TV surgery is also recommended in patients with symptomatic, isolated, severe primary TR, as well as in patients with severe, secondary TR with no signs of relevant right ventricular dysfunction.47 Furthermore, TV repair, whenever feasible is more preferable to TV replacement.47 In the case of patients with prohibitive surgical risk, there are other transcatheter options, such as percutaneous annuloplasty and edge-to-edge valve repair.47
Pulmonary Valve Involvement in Chronic Kidney Disease
Research evidence regarding pulmonary valve involvement in CKD is scarce. Therefore it can be characterised as a neglected area of research that should be further investigated in the future. Pulmonary hypertension (PH) is prevalent in CKD and ESRD patients, and its presence is associated with poor prognosis.69
According to 2015 ESC/European Respiratory Society guidelines for the diagnosis and treatment of PH, PH in chronic kidney failure is attributed to unclear and multifactorial mechanisms (Group 5.4 based on clinical classification).70 More specifically, it may be attributed to fluid overload, high cardiac output due to arteriovenous fistula, lung disease (chronic obstructive pulmonary disease, sleep-disordered breathing), left heart disease and other causes.69
Endocarditis
The risk of endocarditis increases with each advanced stage of CKD and is significantly increased in haemodialysis patients with an annual risk of 1%.71 Endocarditis involving a prosthetic AV is especially high risk, with a mortality of 36%.72 There is a preponderance of staphylococcal infections in ESRD. This is due, in part, to the need for constant intravenous access of these patients. Patients requiring valve replacement surgery have a 1-year mortality of 50%.73
Conclusion
VHD is highly prevalent in patients with renal insufficiency, and its progression specifically accelerates in patients with ESRD, exacerbating the already elevated CVD mortality and morbidity risk. Degenerative AV and MV disease are the most prevalent. Echocardiography is the cornerstone of management for the assessment of severity and haemodynamic consequences, as well as for planning interventions. At present, guidelines for heart valve disease management are based on studies that do not include CKD patients, so patients should be managed with a multidisciplinary team of cardiologists and nephrologists. Endocarditis is more prevalent among CKD patients, with higher mortality than in non-CKD patients, even when valve surgery is performed.
Clinical Perspective
- Although specific guidelines regarding the treatment of patients with valvular heart disease (VHD) and concomitant end-stage renal disease (ESRD) have not been published, valve disease should be assessed more frequently than recommended for the general population with VHD. The rationale for this recommendation is because VHD progresses rapidly in this population and is associated with poor prognosis.
- Given the complexity and the fragility of patients with ESRD and concomitant significant VHD, we should not hesitate to refer patients to more specialised healthcare centres for further investigation due to a potential need for early intervention.