Metabolic dysfunction-associated steatotic liver disease (MASLD) has become the most common cause of chronic liver disease. It is already a global public health issue affecting more than 25% of the global population.1 It is a risk factor for liver cirrhosis; hepatocellular carcinoma; liver decompensation and liver transplantation; extrahepatic manifestations, such as cardiovascular and kidney disease; and extrahepatic malignancies.2–6
Lifestyle plays a prominent role in the development of MASLD. Epidemiological evidence suggests a close relationship with unhealthy lifestyles, which makes lifestyle correction mandatory for all patients with MASLD. Key treatments for MASLD consist of interventions based on physical activity and dietary changes to slow the progression and avoid the development of liver fibrosis.7
There is no pharmacological treatment approved to date for the treatment of metabolic-associated fatty liver disease (MAFLD) in Europe, but there are more than 50 active clinical trials targeting metabolic factors and inflammatory and fibrogenic pathways at the time of writing this review. Until new drugs are approved, all the efforts in clinical practice in the treatment of MASLD patients are directed at motivating lifestyle changes regarding diet and physical activity.
MASLD and Cardiovascular Risk
The prevalence of coexisting conditions associated with MASLD is high, including hypertension, diabetes, obesity and metabolic syndrome, and MASLD is commonly considered as the hepatic manifestation of metabolic syndrome.8
It is well-known that MASLD is associated with a higher risk of developing cardiovascular complications, reaching a 2.6-fold higher risk.9 Indeed, cardiovascular disease (CVD) is the most common cause of death in MASLD patients.10
In the last few years, several studies have supported a strong association between MASLD and a higher risk of certain arrhythmias, such as permanent AF, QTc interval prolongation and ventricular tachyarrhythmias.11 MASLD is also associated with endothelial dysfunction, increased pulse wave velocity, increased coronary arterial calcifications and increased carotid intima-media thickness, which are all established markers for cardiovascular disease.12,13
The mechanisms by which the liver might contribute to this higher cardiovascular risk are complex and heterogeneous. The risk of CVD and other cardiac complications goes in parallel with the severity of MASLD, especially fibrosis severity.14 Indeed, MASLD exacerbates hepatic and systemic insulin resistance, promotes atherogenic dyslipidaemia, induces hypertension and triggers the synthesis of proatherogenic, procoagulant and proinflammatory mediators that may contribute to the development of cardiovascular disease and other cardiac/arrhythmic complications.15
This close relationship between CVD and MASLD raises the necessity of creating awareness of MASLD among other specialists, such as cardiologists, endocrinologists and care providers who treat metabolic diseases.
Guidelines and Recommendations
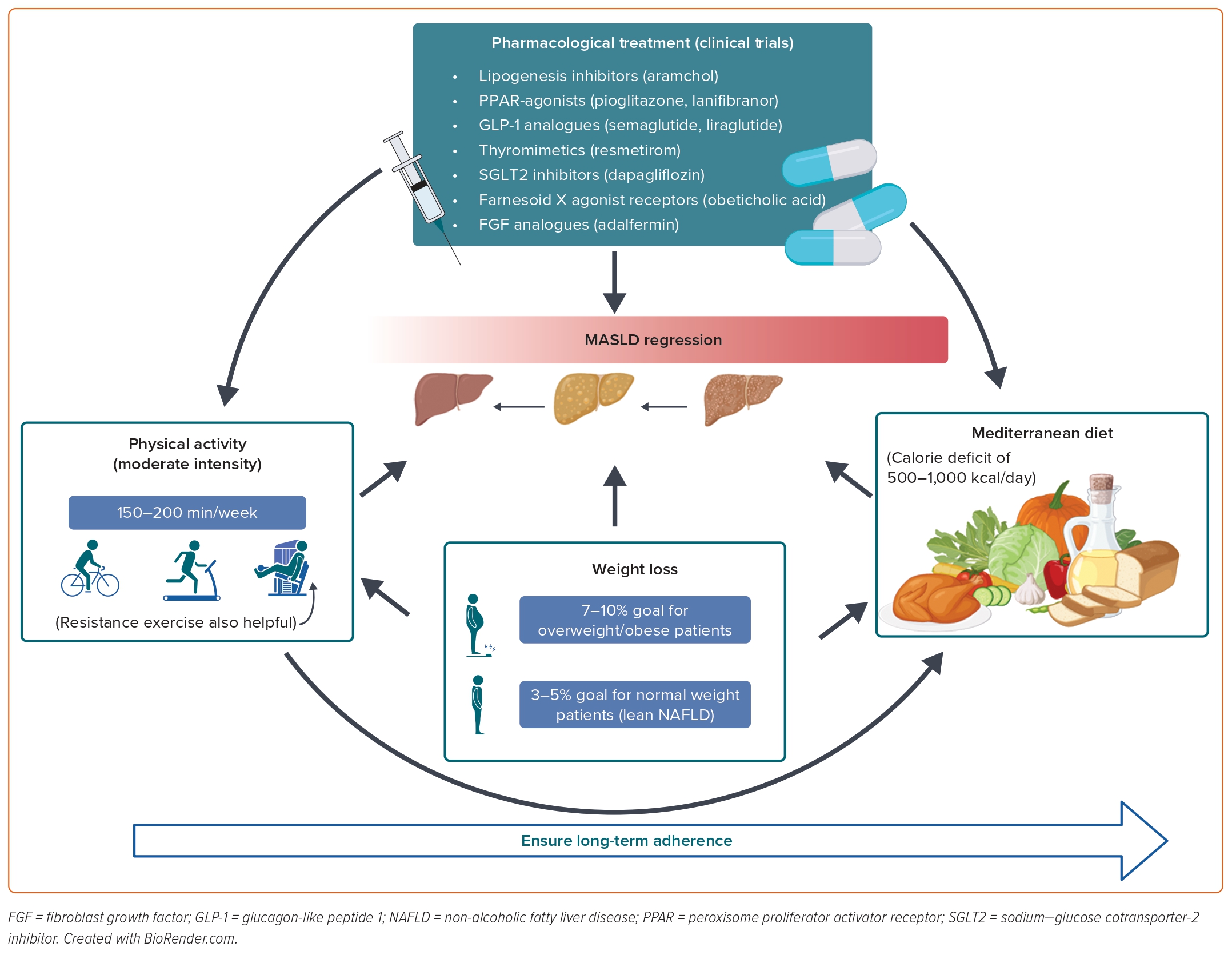
Current clinical practice guidelines agree that the cornerstone of MASLD treatment in obese/overweight patients is a lifestyle intervention involving changes in diet and physical activity, aiming for a weight reduction of 7–10% and a calorie reduction of 500–1,000 kcal/day.16–18 Evidence suggests that calorie restriction can improve numerous metabolic parameters beyond its effect on liver-related outcomes.19 Regarding macronutrient composition, there is a trend toward recommending a Mediterranean diet as it has the most scientific evidence to support it.
Regarding physical activity (PA), moderate-intensity aerobic PA, such as brisk walking or using an exercise bike, in 3–5 sessions for a total of 150–200 minutes per week is generally preferred and recommended. Resistance training is also effective and promotes musculoskeletal fitness, which affects metabolic risk factors and it could be an option for patients with cardiorespiratory deficiency or osteoarticular functional impotence. As PA has a dose-response relationship, vigorous rather than moderate exercise, such as running, carries the full benefit, including for steatohepatitis and fibrosis. Any engagement in PA or any increase in activity is better than continuing to be inactive; thus, both diet and physical activity should be individualised to ensure long-term adherence to this healthier lifestyle.
Weight Loss
Weight loss is the key to improving histopathological features of MASLD. In a meta-analysis of eight randomised controlled trials (RCTs), participants with weight loss ≥7% improved histological disease activity, but this was achieved by less than half of the patients.20
These data have been supported by a 12-month prospective trial with paired liver biopsies in 261 patients. In this trial, a dose-response curve was demonstrated where the greater the degree of weight loss, the more significant the improvement in histopathology, such that 10% weight loss was associated with an improvement in all features of MASLD, including portal inflammation and fibrosis. However, it is important to note that those patients losing 5% of their body weight stabilised or improved fibrosis in 94% of the cases. Unfortunately, only 50% of patients were able to achieve at least a 7% weight loss at 12 months in this trial.21
In a real-world cohort with 2,019 participants, 32% of patients with MASLD who were initially overweight or obese achieved ≥5% weight loss at some time during follow-up but only 25% maintained ≥5% weight loss.22 It is important to note that maintenance of weight loss is an unmet need that should be taken into account during follow-up of MASLD patients.
Although weight loss is the main goal, several systematic reviews have shown that exercise even at low level, without associated weight loss, can reduce intrahepatic lipid (IHL), although this reduction is modest when compared with the effect when there is associated weight loss.23
It has been suggested that patients with MASLD and normal weight (also known as lean-MASLD) have a phenotype of insulin resistance and they are still prone to developing steatosis eased by genetic and gut microbiota-related mechanisms.24
In an RCT involving a 12-month lifestyle intervention programme, a 3–5% weight reduction led to remission of MASLD among 50% of patients with normal weight. Moreover, patients with normal weight were more likely to maintain weight reduction and normal liver enzyme serum levels in the long-term 6-year follow-up (n=154) than patients with obesity.25 Thus, the current updated American Gastroenterology Association clinical practice recommendations suggest a target weight loss of 3–5% in this group of patients.26
Gut Microbiome and its Implication in MASLD
The human intestine harbours a diverse community of microbes that promote metabolism and digestion in their symbiotic relationship with the host. Microbial metabolites produced in a dysbiotic intestinal environment and host factors are important in the pathogenesis of liver disease.27
Gut dysbiosis is a common factor for type 2 diabetes, metabolic syndrome and MASLD.28 The study of the microbiome is still challenging; for example, how to distinguish what is derived from the host and what comes from the microbiota still requires a lot more investigation. Patients with MASLD show increased numbers of Bacteroidetes and changes in the presence of Firmicutes. The proteolytic bacteria, such as Bacilli, Streptococci, Propionibacterium, Clostridium and Bacteroides are associated with the pro-inflammatory responses and progression to MASLD.29
In advanced fibrosis (F3–F4) patients, a decrease in Firmicutes and a greater number of Proteobacteria have been documented.30 Therefore, numerous therapeutic options have been proposed to modulate the intestinal microbiota, including probiotics, prebiotics and faecal microbiota transplantation. The main genera of probiotics studied are Lactobacillus and Bifidobacterium. The proposed mechanisms of actions are reducing intestinal permeability, releasing antimicrobial peptides or preventing the translocation of bacterial products.31
Studies using prebiotics in MASLD are limited compared to probiotics. A meta-analysis of 21 studies including 1,252 participants reported that the administration of probiotics and synbiotics (probiotics and prebiotics) was associated with a reduction in liver stiffness measurement by elastography and steatosis grade by ultrasound.32
However, a later comprehensive meta-analysis concluded that more studies are needed to demonstrate the effects of probiotics, prebiotics and synbiotics in patients with MASLD.33 Last, faecal microbiota transplantation in patients with cirrhosis and alcoholic hepatitis has been proposed; however, studies evaluating the safety of faecal transplantation for MASLD treatment are still scarce.34
Diet Intervention
Macronutrients, such as saturated fatty acids (SFA), trans fats, simple sugars and animal proteins, have a harmful effect on the liver and cardiovascular risk factors. MASLD patients usually have diets that are rich in fizzy drinks, frozen junk food, juice, red meat, lard, processed meats, whole fat dairy foods, fatty snack foods, takeaway foods, cakes and biscuits, and poor in cereals, whole grains, fruit, vegetables, extra virgin olive oil and fish. The benefits of different diet patterns have been studied.
Mediterranean Diet
The Mediterranean diet is the generic name for the traditional dietary patterns of individuals living in the Mediterranean region. It was first defined by Ancel Keys as a diet low in saturated fat and high in vegetable oils.35
The Mediterranean diet varies by country and region, but in general is high on extra virgin olive oil , green leafy vegetables, fruit, whole grains, nuts and legumes. Fish, seafood, dairy and poultry are included in moderation. Red meat and sweets are eaten only occasionally. At the macronutrient composition, 40–50% of total energy intake should come from carbohydrates (mostly complex carbohydrates such as wholemeal bread, pasta and rice), 10–20% from protein (white meats, fish and legumes), and 30–40% from fat, mainly monounsaturated fats and Ω-3 polyunsaturated fatty acids.36 There is strong evidence linking this diet to the improvement of liver steatosis, even in the absence of weight loss.37
The Mediterranean diet protects against MASLD progression and has a beneficial effect on hepatocarcinoma.38 Although the strongest evidence comes from studies with hepatitis viruses-related cirrhosis, closer adherence to the Mediterranean diet appears to protect against hepatocarcinoma.39
Evidence suggests the role of dietary modifications in the prevention and management of cardiovascular risk factors. According to the latest American Heart Association/American College of Cardiology (AHA/ACC) guidelines, patients with CVD should follow dietary recommendations such as sodium reduction, and increasing their intake of vegetables and fresh fruits.40 A systematic review reported that adherence to the Mediterranean diet can improve lipid profile as well as blood pressure, insulin resistance and serum markers of inflammation in patients with diabetes or metabolic syndrome.41 Further, a network meta-analysis indicated that adherence to the Mediterranean diet significantly reduced blood glucose levels, total cholesterol and low-density lipoprotein cholesterol when compared to the control diet.42
For all these reasons, the Mediterranean diet is the only diet recommended as a potential therapy for MASLD by several scientific societies: the European Association for the Study of the Liver, the European Association for the Study of Diabetes and the European Association for the Study of Obesity.16,43
Other Dietary Patterns
Low-carbohydrate Diets
Although long-term studies of clinical outcomes are still lacking, evidence suggests that reducing the consumption of added sugars in the form of industrial fructose may be beneficial for MASLD patients. A reduction in the intake of added sugars, soft drinks and processed foods to a target of less than 2.5% of total calorie intake should be proposed.
Low-fat Diets
Although the evidence is low, patients with MASLD should be advised to reduce their intake of saturated fatty acids, eliminate trans fatty acids and increase their intake of monounsaturated and polyunsaturated fatty acids.44
High-protein Diets
High-protein diets (>20% of total calorie intake) have been advocated for their beneficial effects on weight loss, improvement of glycaemia and lipid blood markers and reduction of hepatic steatosis.45 In addition, high-protein diets promote lean muscle mass retention, an important factor for MASLD patients because sarcopenia is an independent risk factor for MASLD progression.46
Ketogenic Diet
A ketogenic diet consists of restricting carbohydrate intake below 10% of total daily calorie intake (about 20–50 g carbohydrate per day). Although it has been found to be effective for short-term weight loss compared to low-fat diets, its efficacy in MASLD remains controversial.47
Intermittent Fasting
Intermittent fasting involves limiting calorie intake to specific periods of time without changing other components of the diet. A recent systematic review and meta-analysis concluded that intermittent fasting is comparable to continuous energy restriction for short-term weight loss in overweight and obese individuals.48 In this regard, intermittent fasting could improve hepatic steatosis and metabolic markers. Some studies suggest that intermittent fasting is easier to manage compared to a low-calorie diet and may improve long-term adherence to the diet.49
Effect of Different Nutrients on MASLD
There are several macronutrients that deserve special mention when we look at the effect of nutrients in MASLD:
Fructose
Fructose has been associated with insulin resistance, IHL accumulation and hypertriglyceridaemia and CVD.50 Excess fructose consumption (seven drinks per week) has been associated with hepatic fibrosis, independent of calorie intake, in a dose-dependent manner.51 Fructose might provide a more direct substrate than glucose for de novo lipogenesis due to its metabolisation being nearly exclusively limited to hepatocytes.52 Gluconeogenesis from fructose occurs independently of insulin and the energy status of the cell. Thus, fructose (but not glucose-sweetened) beverages have been associated with increased de novo lipogenesis, dyslipidaemia, visceral adiposity and impaired insulin sensitivity.53 Recent RCTs have shown a decrease in liver intrahepatic triglycerides (IHT) after 6–8 weeks of a fructose-restricted diet or one that restricts free sugars in MASLD patients.53,54 So, consumption of fructose is definitely discouraged.
Red and Processed Meats
An increasing number of studies regarding the negative impact of red and processed meat have been published in the past few years. Recent observational longitudinal and cross-sectional studies have linked high consumption of only red meat to an increased prevalence of MASLD. Of note, independent of the consumption of red meat, total white meat, chicken or fish consumption did not show significant associations with MASLD.55–57
Consistently high red and/or processed meat consumption is associated with greater odds for significant fibrosis compared to consistently low consumption.21 Minimising the consumption of red and/or processed meat may help prevent MASLD and significant fibrosis. In addition, cooking meat at high temperatures for a long duration forms heterocyclic amines, which are related to oxidative stress and are associated with insulin resistance. Thus, limiting the consumption of red and processed meat and improving preparation methods may be considered part of MASLD lifestyle treatment.22
Coffee
Coffee enhances the expression of chaperones and antioxidant proteins, such as glutathione, ensuring correct protein folding and degradation in the liver.58 Also, chlorogenic acid, caffeine and kahweol exhibit anti-fibrotic properties by inhibiting hepatic stellate cell activation via down-regulation of the transforming growth factor-β (TGF-β) pathway and inhibiting connective tissue growth factor.59
Two recent meta-analyses showed that although total caffeine consumption is not related to the prevalence of MASLD, regular consumption of coffee with caffeine may significantly reduce liver fibrosis in these patients.60,61 Another meta-analysis has provided a precise quantification of the inverse relation between coffee consumption and the risk of hepatocarcinoma.62
Alcohol
When talking about alcohol, it is noteworthy that not all types of alcohol are the same. Despite a lack of consensus on a specific type of beverage being beneficial to the heart, mounting evidence suggests that ethanol and polyphenols within red wine can synergistically confer benefits against chronic CVDs, mostly ischaemic heart disease.63 In this sense, light to moderate consumption of red wine has been described to be cardiovascular protective due to its polyphenols, such as flavanols, anthocyanin and stilbenes.64
Studies have demonstrated an association between a less serious fibrosis in MASLD patients who have a modest consumption of alcohol.65 Other data suggest that quercetin and resveratrol, two components of red wine, can attenuate multiple profibrotic and proinflammatory gene pathways in mice, and decrease fatty acid availability and reduce oxidative stress, respectively.66,67
However, further studies have ratified alcohol’s harmful effect: analyses show a linear positive association between moderate alcohol consumption and MASLD fibrosis.68 Patients with type 2 diabetes who consume moderate amounts of alcohol had the highest risk of advanced fibrosis, indicating a synergistic effect of insulin resistance and alcohol on the histopathological progression of MASLD.69 In a study involving 8,345 patients with hepatic steatosis who participated in health examination surveys, it was found that even low alcohol intake is associated with increased risks for advanced liver disease and cancer compared to lifelong abstainers. Low-to-moderate alcohol use is associated with reduced mortality and cardiovascular risk but only among people who had never smoked, this study found.51
A review of experts on this issue has recently been published and whether there is a safe amount of alcohol in the general population is a matter of intense debate.
Alcohol can be a cofactor for liver disease progression and intake should be assessed on a regular basis. In the era of personalized medicine, recommendations on how much alcohol may be beneficial for patients can be determined by the degree of fibrosis found in individual patients. Thus, in patients with low to mild fibrosis (F1–F2), a modest consumption of alcohol (≤1 drink per day for women and ≤2 drinks per day for men; one drink equals one bottle of regular beer (12 ounces), one glass of wine (5 ounces), or one shot of liquor or spirits (1.5 ounces)) seems to be liver-safe as in the general population, while alcohol consumption must be completely discouraged in patients with significant and advanced fibrosis or cirrhosis (F3–F4).70
Physical Activity
Sedentary behaviour and low levels of PA are leading risk factors of non-communicable diseases and a major public health problem due to a large proportion of the world’s population remaining physically inactive despite of scientific evidence and recommendations. New actions, strategies, guidelines and recommendations promoted by health authorities encourage people to be active.71,72
Sedentary behaviour is defined as ‘waking behaviour at low energy expenditure ≤1.5 metabolic equivalents (METs) while in a sitting, lying or reclining posture’, such as watching television.73 Sedentary behaviour is not only associated with obesity and MASLD, but also negative health outcomes.74 Interestingly, TV viewing time is independently associated with a higher fatty-liver-index.75 In a population-based study including 1,899 adults, long total and prolonged sedentary time were associated with increased likelihoods of MASLD, whereas having more breaks per sedentary hour and reallocating sedentary time to light-intensity PA was associated with reduced likelihoods of MASLD.76 Even in those patients with MASLD-related advanced hepatic fibrosis or cirrhosis, regular physical activity has been shown to reduce portal pressure, improve frailty, sarcopenia and quality of life.77,78
Other approaches, such as participation in structured lifestyle intervention programmes and/or enrolment in clinical trials for the treatment of MASLD, may increase the potential for adherence. There is also interesting data about a protective effect on carcinogenesis obtained from epidemiological studies reporting a lower incidence of hepatocarcinoma between the groups with the most frequent physical activity.79
Differences between physical activity carried out in leisure time and occupational physical activity have been described regarding cardiovascular effects. In a population study involving 104,046 participants aged 20–100 years in the Copenhagen General Population study, data suggested that leisure time PA is associated with a reduced risk of CVD and all-cause mortality, while these relationships for occupational PA are not apparent.80
The evidence regarding these differences in MASLD patients is weaker, but there is a study with 21,015 participants, 4,942 of them with MASLD, where those with ≥150 minutes per week of recreational activity had a reduced risk of MASLD, whereas ≥150 minutes per week of travel or work activity did not show this reduction. Thus, physical activity should be differentiated by domains when managing MASLD.81
Exercise
Exercise is a subset of PA that is planned, structured and repetitive and has a final or intermediate objective of improving or maintaining physical fitness. Even at 150 minutes per week, exercise without associated weight loss can produce about 20–30% relative reduction in IHL, which is modest when compared with a reduction induced by weight loss.23
Aerobic Exercise
In a short-term 8-week intervention, there were no differences in efficacy on IHL reduction found if the exercise programme was either higher intensity or of longer duration – both being about 30%.82 In a long-term (12-month) intervention, both vigorous (defined as not being able to say more than a few words without pausing for a breath, for example running) and moderate (defined as being able to talk but not sing while performing the physical activity, for example brisk walking) exercise (30 minutes, five times per week) were equally effective in reducing IHL content, most likely mediated by weight loss, when present; therefore, it was suggested that moderate-intensity training would be good for prevention and treatment of MASLD, because the target (IHL reduction) was reached at 6 months and also persisted over time (12 months).83 On the other hand, another study showed that MASLD patients who engaged in at least 150 minutes per week, of which 50% was vigorous, obtained a significant mortality benefit.84
A recent meta-analysis studied several heterogeneous aerobic exercise interventions (130–220 minutes per week and high-intensity or moderate-intensity) showing a clear correlation between IHL changes and weight and hepatic enzymes (alanine aminotransferase and aspartate aminotransferase).85
Resistance Exercise
Resistance exercise improves MASLD with less energy consumption. Thus, resistance exercise, such as push-ups, sit-ups and weight-lifting, may be an option for MAFLD patients with poor cardiorespiratory fitness or for those who cannot tolerate or participate in aerobic exercise, such as those with osteoarthritis of the knee. These data may indicate a possible link between resistance exercise and lipid metabolism in the liver.86
Invasive Treatments
When lifestyle intervention does not achieve the goals, a wide range of invasive procedures are possible, from endoscopic techniques to bariatric surgery. Endoscopic bariatric and metabolic surgery procedures are promising less-invasive options: endoscopic intragastric balloons have shown significant metabolic and histological improvements in MASLD patients in a prospective study comprising 21 patients.87,88 Endoscopic sleeve gastroplasty has been shown to improve liver parameters, induce weight loss, and reduce HbA1c levels in patients with MASLD.89 However, long-term safety and efficacy data are needed.
Evidence is more robust regarding bariatric surgery. Currently accepted criteria for bariatric surgery are BMI ≥40 kg/m2 irrespective of metabolic comorbid disease or BMI ≥35 kg/m2 with comorbidities, such as type 2 diabetes or pre-diabetes or uncontrolled hypertension. MASLD is increasingly accepted as a comorbid condition that would benefit from bariatric surgery.90 Bariatric surgery provides positive and sustainable effects in terms of weight loss. Its effectiveness and safety depend fundamentally on the type of technique and the experience of the centre.91 Vertical gastrectomy and gastric bypass provide higher weight loss rates than gastric banding; however, they also have higher rates of complications and mortality.
In general, bariatric surgery cannot be considered a primary therapy for the treatment of compensated MASLD cirrhosis; however, it seems to be safe in carefully selected patients in the setting of liver transplant or research protocol in specialised centres.
Pharmacological Treatment
Numerous drugs with different targets have been developed in the past 15 years for MASLD. Many of them are in development or preclinical studies or have already failed to show improvement in steatohepatitis features.
The goal of the emerging drugs is the reduction of fatty acid accumulation, reduction of inflammation and regression of fibrosis. The Food and Drug Administration (FDA) and European Medicines Agency describe MASLD resolution as the presence of any grade of steatosis, no ballooning and only minimal (grade 1) lobular inflammation and – at the same time – no worsening of the stage of fibrosis; or the improvement of fibrosis by at least one stage without any worsening of steatohepatitis (no worsening of ballooning and lobular inflammation and a one-grade change in steatosis may be acceptable).
Lipogenesis Inhibitors
Aramchol, an inhibitor of stearoyl-CoA desaturase, is the most studied drug targeting lipogenesis inhibition. However, aramchol did not meet the primary endpoint – a significant reduction of IHL – of the phase II trial.92 A phase III trial is ongoing and the main endpoints are the improvement in liver fibrosis greater than or equal to one stage and no worsening of steatohepatitis.
Omega-3 polyunsaturated fatty acids (ω-3 PUFA) include α- linolenic acid and its metabolites eicosapentaenoic and docosahexaenoic acids. Recent meta-analyses have found that ω-3 PUFA significantly decreases liver transaminases, liver fat and insulin resistance, having no effect on body weight in MASLD.93 There are also artificial ω-3 PUFAs, icosabutate (NST-4016), being studied in a phase II trial in patients with biopsy-confirmed non-alcoholic steatohepatitis (NASH). Interim analysis data has indicated improvements in non-invasive fibrosis and inflammatory biomarkers.94
Peroxisome Proliferator-activated Receptor Agonists
Pioglitazone, a selective peroxisome proliferator-activated receptor γ (PPARγ) agonist, did not achieve the primary endpoint of a two-point reduction in non-alcoholic fatty liver disease (NAFLD) activity score (NAS) without worsening fibrosis in the PIVENS trial.95 Several adverse events, such as fluid retention, weight gain and bone loss, have led to questions about its long-term use in NASH. Its use in clinical practice has been replaced by newer insulin-sensitising agents (sodium–glucose cotransporter-2 [SGLT-2] inhibitors and glucagon-like peptide 1 [GLP-1] receptor agonists), with more pronounced effects on weight loss and cardiovascular benefits.
Lanifibranor is a triple PPARα/γ/δ agonist. Lanifibranor was well tolerated and the percentage of patients with meaningful improvements in steatosis, activity and fibrosis scores was significantly higher in the lanifibranor-treated arms in a completed phase IIb study with 247 patients.96 Two more trials to evaluate the efficacy of lanifibranor in concomitant MASLD and type 2 diabetes and advanced fibrosis due to NASH are ongoing.
Glucagon-like Peptide 1 Receptor Agonists
GLP-1 receptor agonists are indicated and accepted by the FDA for obesity and type 2 diabetes for children and adults.
Semaglutide treatment achieved the highest response rate in NASH resolution in a trial to date without worsening of fibrosis in the recently completed 72-week phase II trial.97 However, there was a lack of fibrosis reversal despite the massive weight loss, so there is the question whether the effects are independent of weight loss.
Liraglutide has demonstrated a hepatitis activity reduction and fibrosis reduction in a phase II study.98 In a meta-analysis of eight clinical trials, it was shown that GLP1R agonist could improve histology in patients with type 2 diabetes and MASLD and liver function with a reduction of BMI, liver fat concentration and glycaemia levels.99
In addition, dual agonists are being studied for MASLD treatment, associating GLP-1 with glucose-dependent insulinotropic polypeptide (GIP) agonists, such as tirzepatide.100 In a phase III double-blind RCT, tirzepatide showed weight loss up to 20.9% with doses up to 15 mg once weekly compared with 3.1% with placebo, and absolute reduction in liver fat content of 8.1%.101
Thyromimetics
Resmetirom is the first oral, liver-directed thyroid hormone receptor-β1-selective agonist. In a 36-week phase II randomised clinical trial, resmetirom achieved NASH resolution in a subset of patients with control biopsies. Liver steatosis and liver stiffness improved together with lipid serum profile and fibrosis biomarkers, such as Pro-C3 and hepatic enzymes, whereas a significant reduction in NAFLD activity was observed.102
Sodium–Glucose Cotransporter-2 Inhibitors
Although the role of SGLT-2is in the treatment of MASLD is limited by a small sample size and lack of histological outcomes, current data suggest that SGLT-2i improve metabolic risk factor and liver fat content in patients with MASLD.103 Despite the limitations in available data on MAFLD/NASH, their use for the treatment of diabetes may be beneficial for patients needing an improvement in glycaemic control and optimising the cardiometabolic risk factors associated with MAFLD/NASH.104
Farnesoid X Receptor Agonists
Obeticholic acid (OCA) is a first-in-class farnesoid X receptor (FXR) agonist approved by the FDA for non-cirrhotic primary biliary cholangitis treatment. It is close to being approved for liver fibrosis in MASLD as it induces histological regression of fibrosis compared to placebo in non-diabetic pre-cirrhotic MASLD patients.105 In a phase III trial, 14.9% of MASLD patients with F1–F3 fibrosis improved NASH without worsening fibrosis.106
OCA is not exempt from side-effects, such as pruritus and an increase in LDL, so the FDA has delayed conditional approval until more efficacy and safety data are available, mainly concerning the increase of LDL and its possible cardiovascular effect. Second-generation FXR agonists, such as MET409, tropifexor or cilofexor, are in development with the aim of avoiding these side-effects.
Fibroblasts Growth Factor Analogues
Aldafermin is an engineered fibroblast growth factor (FGF)19 analogue studied in MASLD patients with liver fibrosis stage 2 or 3. Fibrosis improvement (≥1 stage) with no worsening of NASH was achieved in 38% of patients receiving aldafermin versus 18% of patients receiving placebo (p=0.10).107 New trials are ongoing to determine whether aldafermin improves liver fibrosis in NASH subjects with compensated cirrhosis.
Galectin Antagonist
Although the involvement of galectin in chronic liver disease remains controversial, it seems that its increased expression is linked to accelerated cirrhosis development and worsening of liver function.108 Belapectin is an inhibitor of galectin-3 that has been evaluated in cirrhotic MASLD patients with portal hypertension. In a 52-week phase IIb study, belapectin did not change fibrosis or NAFLD activity, but a significant reduction of hepatic venous-portal gradient and oesophageal varices development was observed.109 A new phase II/III trial has been initiated to evaluate belapectin in patients with liver cirrhosis due to MASLD and clinical signs of portal hypertension but without oesophageal varices at baseline.
Conclusion
The key cornerstone of MASLD treatment is based on lifestyle recommendations regarding diet, mostly adopting a Mediterranean diet until we have stronger evidence about other diet patterns; and encouraging an increase in physical activity (Figure 1). As MASLD is associated with a high cardiovascular risk, treating patients with MASLD will reduce CVD-related mortality, which is indeed the most common cause of mortality in these patients.
Despite the race to find new pharmacological treatments, lifestyle recommendations are fundamental in these patients and should be the first approach in chronic patients where the prescription should follow a specific algorithm, evaluating the individual’s habitual diet habits, physical activity, physical function, health status, exercise response, stated goals and preferences. 










