One of the greatest achievements of public health in the twentieth century has been the almost doubling of life expectancy in the Western world. Yet this now ageing population brings new challenges, as the prevalence of little-understood geriatric conditions increases, together with the rising prevalence of age-related disorders, such as syncope.
The definition of syncope, as outlined by the European Society of Cardiology (ESC), is a transient loss of consciousness (T-LOC) due to global cerebral hypoperfusion characterised by rapid onset, short duration and spontaneous complete recovery.1 Previous variations in the definition of syncope have led to its prevalence being poorly appreciated.2 By distinguishing syncope/T-LOC from other causes of loss of consciousness (for example, epileptic seizure, concussion), the present definition aims to minimise conceptual and diagnostic confusion.1
Why is syncope in the elderly important? Presentation in this age group is challenging and often recognition is the first step to optimising management and care of these patients. To start with, syncope in the older patient is under-recognised, particularly in acute care settings because the presentation is frequently atypical.
The older patient is less likely to have a warning or prodrome prior to syncope, commonly has amnesia for loss of consciousness and frequently experiences an unwitnessed event,3 thus presenting with a fall rather than T-LOC.4–6 These events are typically described as non-accidental (not a trip or slip) or unexplained falls. Therefore, history alone cannot be relied upon when assessing the older patient. Injurious events such as fractures and head injuries, are also more common, further emphasising the importance of thorough early investigation and diagnosis.3
There is an increased susceptibility to syncope with advancing age that is attributed to age-related physiological impairments in heart rate (HR), blood pressure (BP), cerebral blood flow and neurohumoral stability.7 This, combined with multi-morbidity and polypharmacy in these complex patients adds to their vulnerability.7 Furthermore, cardiac causes are more common as patients age.8 Emerging evidence has proposed consideration of early insertion of patient-activated internal loop recorder (ILR) devices in this age group.9,10
In the older patient, syncope is a major cause of morbidity and mortality and is associated with enormous personal and wider health economic costs.7 Quality of life studies have consistently shown that functional impairment induced by syncope is similar to that of chronic diseases such as rheumatoid arthritis and epilepsy11–13 underscoring the significant morbidity attached to syncope.
The purpose of this review is to highlight the characteristics and epidemiology of syncope in the older person.
Epidemiology
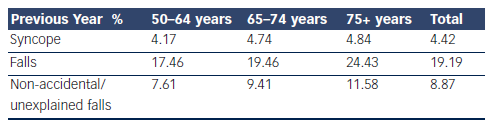
The Irish Longitudinal Study on Ageing (TILDA [www.tilda.ie]) is a population-based study of adults 50 years and over that incorporated questions on syncope and falls in addition to a broad spectrum of health, social and economic questions. A number of community dwelling adults (8,163), mean age 62, range 50–106 years, were asked whether they experienced fainting in their youth, throughout their life or over the past 12 months. A total of 23.6 % had one or more episodes in the previous 12 months of which 4.4 % were syncope and 19.2 % were falls (see Table 1). Although the prevalence of syncope rose with age, the increase in falls was much more remarkable, in particular the increase in non-accidental or unexplained falls was most striking. Unwitnessed syncope most commonly presents as non-accidental or unexplained falls, supporting the rising prevalence of atypical syncope with advancing years.
The General Practitioners’ Transition Project in the Netherlands demonstrated that the age distribution of patients presenting to their GP with syncope shows a peak in females at 15 years of age and a second peak in older patients (see Figure 1).14 The Framingham Offspring study similarly demonstrates a bimodal peak of first syncope in mid-teens and over 70 years.15
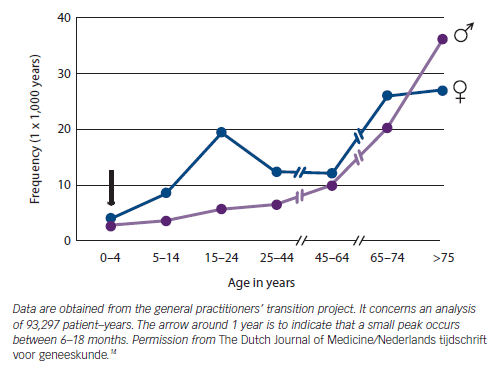
The true prevalence of syncope is underestimated due to the phenomenon of amnesia for T-LOC. Amnesia has been reported in patients with vasovagal syncope (VVS) and carotid sinus syndrome (CSS),3,16 but is likely to be present in all causes of syncope. The overlap between syncope and falls also leads to under-reporting.6
Causes of Syncope in the Elderly
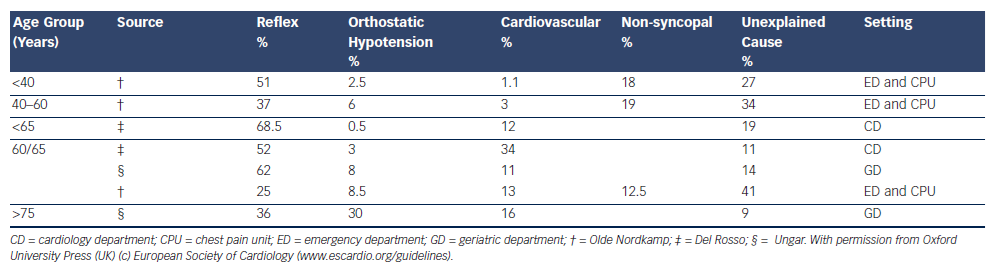
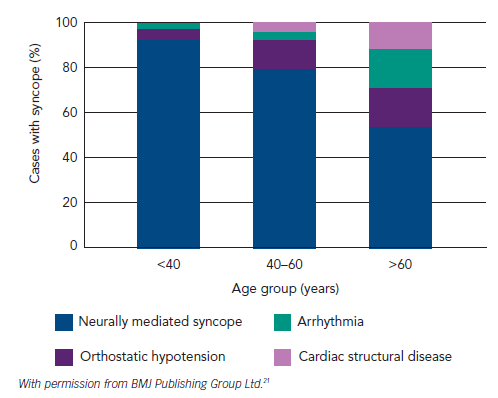
Reflex syncope and orthostatic hypotension (OH) are the most frequent causes of syncope in all age groups and clinical settings, and responsible for the majority of episodes in younger patients. However, cardiac causes of syncope, structural and arrhythmic, become more common in older patients and are responsible for one-third of syncope in patients attending the Emergency Room and Chest Pain Unit1,17–19 (see Table 2,17–19Figure 220 and Figure 3.21)
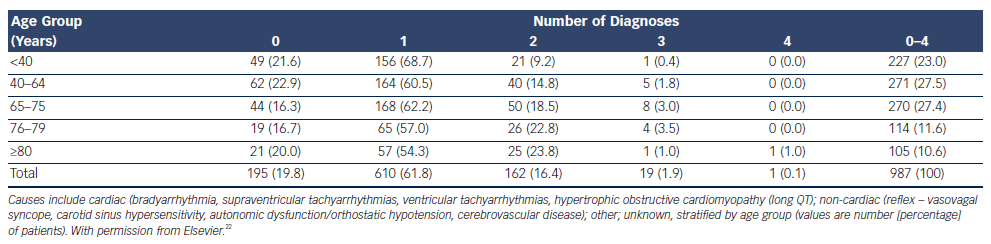
The prevalence of unexplained syncope varies according to diagnostic facilities and age from 9 to 41 % (see Table 21,17–19). In the older patient, history may be less reliable and multiple causes of syncope may also be present (see Table 3).4,18,22–24 Multi-morbidity and polypharmacy are more common in older patients with syncope and can add to the complexity of identifying an attributable cause of events.25
Approach to the Older Person Presenting with Syncope
Classification
Syncope is classified as reflex/neurally mediated syncope, syncope secondary to OH and cardiac syncope.1
Assessment
Initial Evaluation
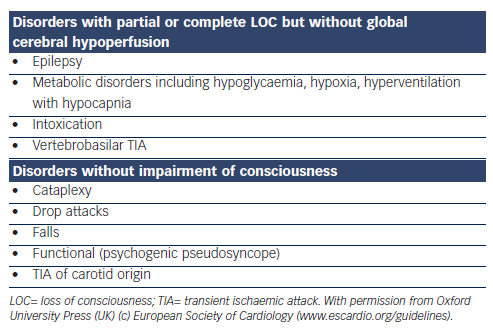
Initial evaluation should establish whether T-LOC occurred, whether aetiology has been identified and whether there is any evidence of a high risk of cardiovascular events or death.1,21 The initial evaluation in patients over 50 years includes detailed history, collateral history, driving history, physical examination, 12-lead electrocardiogram (ECG), measure of orthostatic BP, routine blood tests and CSM. Where possible, the history should elicit ‘the 3 Ps’ (provokers, prodrome, posture), as well as circumstances leading to the event, description of the event, duration of the event, details of the recovery phase and a thorough medication history,1 coupled with a witness account. History alone cannot be relied upon in the older population as commonly these patients (28 % in one study3) experience amnesia for loss of consciousness.3,18 In 50 % of cases of syncope in the older population an accurate collateral history is not available.6 The differential diagnosis of syncope most frequently includes epilepsy, strokes and transient ischaemic episodes and falls (see Table 4).1 The absence of T-LOC is important in differentiating between syncope and ‘drop attacks’, which are defined as a loss of postural control when the patient falls without loss of consciousness but with difficulty in resuming the erect position after the event.26
If syncope remains undiagnosed, further investigation is necessary, including in head-up tilt (HUT), cardiac investigations and ambulatory BP monitoring.24,27 In some studies up to 30 % of older patients with syncope have more than one possible attributable cause, emphasising the necessity for a full comprehensive assessment in the older patient.22,28
1) Reflex Syncope/Neurally Mediated Syncope
Vasovagal Syncope
VVS is a neurally mediated reflex in which there is a relatively sudden change in autonomic nervous system activity leading to a fall in BP, HR and cerebral perfusion.29 In young patients a diagnosis can usually be made from history alone. This is not always the case with older patients. Although VVS is the most common cause of syncope in the older patient, it may not follow the benign course commonly observed in the young.30,31 The classic prodrome usually described as pallor, sweating, nausea and dizziness may be shorter in duration or in some instances non-existent or poorly recognised in the older patient.30,32–34 In the older person, VVS is more likely due to a dysautonomic response representing an inability of the baroreflex to adapt to physiological challenges, which results in a progressive fall in HR ± BP before the onset of symptoms.35 VVS has multiple triggers including a warm environment, prolonged standing, dehydration especially in those on diuretics or anti-hypertensive and vasodilator medications.34
Investigation
HUT testing is well tolerated in the older patient34 and is indicated in syncope of unknown origin,36 when history is atypical, driving is a concern, or serious injury sustained,37 and as outlined in the ESC guidelines.1 Even the older, frailer patient with cognitive impairment tolerates HUT,38 including both passive and GTN-provoked HUT.39,40
The HUT is positive when there is induction of either reflex hypotension/bradycardia or delayed OH associated with syncope or pre-syncope1 with symptom reproduction. In unexplained falls due to reflex syncope, patients may deny witnessed loss of conscioussness induced by HUT. This was the case in 42 % of older patients in one study.3 Responses are vasodepressor (hypotension), cardioinhibitory (bradycardia) or mixed.41 An exception to the classification is chronotropic incompetence, where the patient has no compensatory rise in HR on HUT.24
If HUT results in a cardio-inhibitory response and syncope, capture of a real-time event with early insertion of an ILR9 should be sought.42 It is also important to remember that HUT does not always replicate real-time syncopal episodes as has been demonstrated in ILR analysis in patients with VVS.43
Implantable Loop Recorder – Use in Reflex Syncope
The International Study on Syncope of Unknown Etiology (ISSUE-2)44 trial provided evidence that early ILR insertion to capture syncope in real-time in those with suspected reflex syncope, ensured safe and effective directed therapy in patients experiencing frequent syncope.44 The mean age of trial participants was 66 ± 14 years. Other characteristics of participants were syncope beginning in middle or older age, frequent injury and short prodrome. The study demonstrated a reduction in recurrent syncope rates following ILR-guided therapy ie. pacemaker insertion, following asystole or bradyarrhythmia.44 Fifty per cent of those with recurrent unexplained syncope had asystole during symptoms.44
In the ISSUE-3 trial, patients who were ILR positive (documented asystolic episode) but had negative tilt tests, had the best outcomes from cardiac pacing with a 5 % recurrence of syncope at 2 years.45 However, 25 % of those who were ILR positive and actively paced had a recurrence of syncope at 2 years, when those with positive and negative tilts tests were included.45,46 The study raised questions about the origin of the asystole in the older pacemaker group and whether reflex syncope or age-related conducting tissue disease was responsible.47
Management of Vasovagal Syncope
Cardiac and psychotropic medications can cause hypotension and VVS, therefore initial treatment focuses on modification of culprit medications (up to 40 %). Management includes education with advice on adequate fluid intake,48 physical counter manoeuvres (PCM),7 compression stockings, tilt training49 and feedback to patients of haemodynamic changes correlating with symptoms at the time of HUT.
Older patients with VVS are more likely to require cardiac pacing, for example, when spontaneous cardioinhibitory response in the setting of frequent syncope is observed.1 Cardiac pacing in VVS is given a class IIa recommendation in international guidelines, in those over 40 years with recurrent reflex syncope and documented spontaneous cardioinhibitory response during monitoring; a Class IIb recommendation for refractory symptoms in the same age group in the presence of a documented cardio-inhibitory response on HUT.1,50 The ISSUE-3 trial refines this to include those VVS patients over 40 years, with syncope beginning in middle or older age,51 with three or more episodes of syncope in the previous 2 years and spontaneous asystole during monitoring.46,47 These patients correspond with those defined by the ESC guidelines as patients with high risk of injury or high frequency of syncope recurrence.1,51
Carotid Sinus Hypersensitivity and Carotid Sinus Syndrome
CSS is exclusively a disorder of ageing and current guidelines advise that carotid sinus massage (CSM) should be performed in patients over 40 years with unexplained syncope.1 Careful history taking may reveal triggers such as head turning, tight collars, shaving and vagal stimuli,24 although micturition, defaecation and known triggers of VVS can also provoke CSS. Contraindications to CSM include transient ischaemic attack/stroke within 3 months,1 recent myocardial ischaemia52 or evidence of carotid bruit1 unless significant stenosis has been excluded by carotid dopplers. Using the exclusion criteria, the risk of stroke or transient ischaemic attack (TIA) from CSM has been reported as one per 1,000 episodes of massage.27
Traditionally, carotid sinus hypersensitivity (CSH) has been defined as a ventricular pause lasting >3 seconds and/or a fall in systolic BP of 50 mmHg during CSM without spontaneous syncope.1 CSS is diagnosed when the above criteria is associated with spontaneous syncope.1 The CSH response is categorised as cardio-inhibitory, vasodepressor or a combination of both.27,45 Recently, authors proposed new criteria for exaggerated responses to CSM-asystole ≥6 seconds and a fall in mean arterial BP ≥60 mmHg over 6 seconds53 based on data from a population study where the 95th percentile for CSM response was 7.3 seconds of asystole and 77 mmHg drop in systolic BP.8 Wieling et al.54 observed that there was no LOC before 6 seconds of asystole providing a pathophysiological reason to extend the guidelines.54
CSH may be an epiphenomenon of ageing rather than a disease process given that it is evident in up to 35 % of asymptomatic community-dwelling older people.8 Recently, CSH has been associated with cognitive impairment and dementia; however, it is not clear whether it is a risk factor for development of dementia or consequence of neurodegenerative pathology.55,56
Investigation
CSM should be performed in all patients over 40 with syncope of unknown aetiology1 and unexplained falls.7
Management of Carotid Sinus Hypersensitivity and Carotid Sinus Syndrome
Although the most common presentation of CSS is syncope, patients can also present with falls and drop attacks.52,57 The ESC guidelines only advise pacing with regard to syncope in CSS.1 The American Geriatrics Society guidelines on falls prevention in older adults recommend cardiac pacing for CSH and unexplained/non-accidental falls.58 Dual-chamber permanent pacemaker insertion for cardio-inhibitory or mixed subtypes of CSS is the treatment of choice.59
2) Orthostatic Hypotension
OH is defined as a reduction in systolic BP of at least 20 mmHg or in diastolic BP of at least 10 mmHg within 3 minutes of standing.60 Orthostatic intolerance (OI) refers to symptoms and signs with upright posture due to circulatory abnormality.1 Syndromes of OI that may cause syncope as per ESC guidelines include: initial OH where symptoms of lightheadedness/dizziness or visual disturbance are experienced seconds after standing; classic OH where dizziness, pre-syncope, fatigue, weakness, palpitations, visual and hearing disturbances are experienced; delayed OH where there is a prolonged prodrome frequently followed by rapid syncope; delayed OH and reflex syncope where there is a prolonged prodrome always followed by syncope; reflex syncope triggered by standing where there is classic prodrome and triggers always followed by syncope and postural orthostatic tachycardia syndrome where there is symptomatic HR increases and instability of BP without syncope.1 Many older patients with OH also have postprandial hypotension.
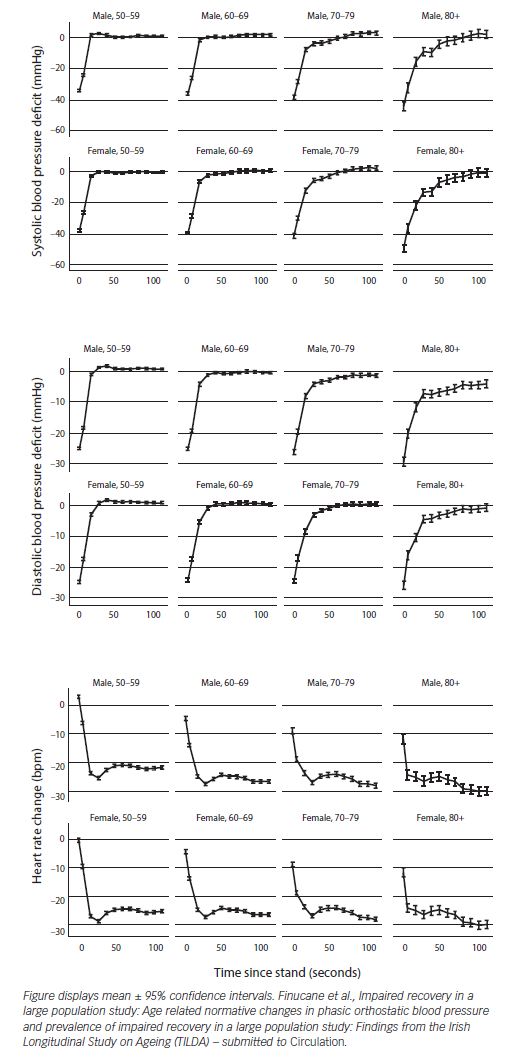
Prevalence of OH in the older-aged community-dwelling adults is 30 %61 and increases to more than 50 % in geriatric ward patients62 making its diagnosis highly relevant. Causes of OH include volume depletion or disturbance of the autonomic nervous system resulting in failure in vasoconstrictor compensatory mechanisms induced by upright posture.63 The prevalence is higher if phasic BP measures are used. In the TILDA population, 50 % of people over 70 years had persistent OH up to 40 seconds after standing. Failure of BP to return to baseline values is associated with adverse physical and cognitive outcomes such as syncope, falls, depression and cognitive dysfunction (Finucane, Impaired Orthostatic Blood Pressure Recovery is Independently Associated with an Increased Risk of Falls in Older Community Dwelling Adults; submitted).
The TILDA study has also shown that supine systolic hypertension (SSH) coupled with OH is a risk factor for cognitive impairment and depression.64 There is a striking age gradient in impaired orthostatic BP response as evidenced in Figure 4 (Finucane et al., Age related normative changes in phasic orthostatic blood pressure and prevalence of impaired recovery in a large population study: Findings from the Irish Longitudinal Study on Ageing (TILDA) – submitted to Circulation).
Causes of Orthostatic Hypotension
The following are the most common causes of OH in elderly people:
- medication induced (commonest cause in the older person);
- primary autonomic failure associated with Parkinson’s disease, multisystem atrophy etc.; and
- secondary autonomic failure for example secondary to diabetic neuropathy or alcoholic autonomic failure and dehydration.
Differential diagnosis must also include anaemia and diagnosis of its underlying cause, Addison’s disease or malignancy.
Medication-induced Orthostatic Hypotension
Syncope due to OH is linked to the use of vasoactive medications, most commonly diuretics and nitrates.63 Many medications cause OH: cardiovascular medications such as alpha-blockers, diuretics, nitrates, neurological medications, anti-parkinsonian, anti-depressant medications and benzodiazepines.28,60,65 Multivariate analysis in one recent study found that predictors of OH were varicose veins and treatment with alpha-receptor blockers, nitrates or benzodiazepines.28
Investigation of Orthostatic Hypotension
Active standing or passive and unprovoked HUT resulting in symptoms coinciding with a 20 mmHg systolic or diastolic BP drop of 10 mmHg within 3 minutes of orthostatic stress confirms a diagnosis of OH.60 BP and HR usually recover to baseline values within 30 seconds but this reflex increase changes with age and haemodynamic recovery is delayed in elders (see Figure 4, Finucane et al.)
Ambulatory BP monitoring in OH patients guides assessment of diurnal variation in BP,1 postprandial hypotension, response to timing of medications and nocturnal supine hypertension.
Management of Orthostatic Hypotension
Culprit medications should be modified or eliminated and conservative measures adopted prior to consideration of additional pharmacotherapy.66 Conservative measures include PCM,67 enhanced fluid and salt intake, elevation of the head of bed when sleeping (modifies neuroendocrine control of nocturnal polyuria, redistribution of body fluids and supine hypertension),68,69 compression stockings, abdominal binders70,71 and rapid ingestion of cool water for symptoms of OI and post-prandial hypotension.72
Medications, such as midodrine (an alpha 1 agonist)73–75 and fludrocortisone (a mineralocorticoid that stimulates renal sodium retention and expands intravascular volume), are both well tolerated in the older patient in moderate dosages.68 Pyridostigmine, octreotide and desmopressin are adjunct therapies but are less well researched in older patients and less well tolerated in our experience.
The combination of SSH and OH poses challenging management decisions as treatment of SSH may worsen symptoms of OH.76 Attention to symptom relief is balanced with treatment of vascular risk associated with SSH.77,78 TILDA researchers reported that beta blockers and anti-depressants were risk factors for OH in people over 50 with SSH.78 Other cardiovascular medications were not associated with OH in people with SSH.
3) Cardiac Syncope
One-third of cases of syncope in the older patient are caused by cardiac disorders18 (see Figure 3).21 There is a higher morbidity and mortality associated with cardiac syncope.15,79 Cardiac syncope is characterised by little or no prodrome, occurrence when supine or during exercise and association with palpitations or chest pain.7 However, the older patient may not recall these symptoms. Heart disease is an independent predictor of cardiac syncope – sensitivity 95 % and specificity 45 %;80 the prevalence of cardiac disease, including structural heart disease and arrhythmias, rises dramatically with age as detailed in Figures 2 and 3.20,21,81 Cardiac syncope should be considered when the surface ECG is abnormal or left ventricular systolic dysfunction is present.7
Investigation
The gold standard for the diagnosis of cardiac syncope is symptomrhythm correlation i.e. contemporaneous HR and rhythm recording during syncope. Cardiac monitoring may also identify diagnostic abnormalities, such as asystole in excess of 3 seconds and rapid supraventricular (SVT) or ventricular tachycardia (VT).82–84 The absence of an arrhythmia during a recorded syncopal event excludes arrhythmia as a cause unless the patient has a dual diagnosis. In patients over 40 years with recurrent unexplained syncope who do not have structural heart disease or abnormal ECG, the attributable cause of syncope is bradycardia in over 50 %.44,85–87
Cardiac Monitoring
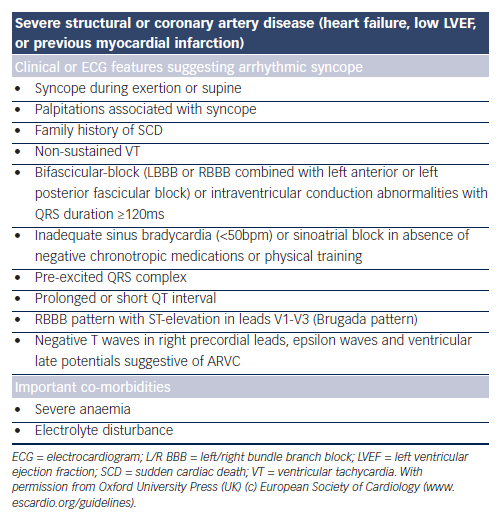
Prompt hospital admission or intensive monitoring is recommended when cardiac disease is present in the setting of syncope (see Table 5).1 Although telemetry or in-patient monitoring is indicated if the patient is at high risk of a life-threatening arrhythmia as per ECG abnormalities detailed in Table 5, the diagnostic yield from telemetry is low –16 % in one series.88 Holter monitoring is only indicated if a patient is experiencing episodes of sufficient frequency to detect an abnormality up to 72 hours of recording.1 Diagnostic yield from Holter monitoring is only 1–2 % in unselected populations.1 Incidental arrhythmias are much more common in older persons, for example, atrial fibrillation occurs in one in five men over 80 years.89
External loop recorders have a higher diagnostic yield in older patients; however, some older patients may have difficulty operating the devices,90,91 therefore automated arrhythmia detection is preferred.92 Normal ambulatory ECG (Holter or external loop or otherwise) in the absence of symptoms does not exclude a causal arrhythmia7 and monitoring for longer intervals is imperative to capture rhythm during symptoms.
Diagnostic rates are much higher in older patients using the ILR,93,94 up to 50 % in patients with syncope and unexplained falls.9,10,95 Early insertion of ILRs in the older person is important to consider in view of the disproportionately high number of cardiac causes of syncope in this group.9 This approach is also more cost-effective.96,97 Difficulties with ILRs include inability to activate the device, particularly if patients have cognitive impairment, however, automated recordings and remote monitoring have much improved diagnostic yield.42 Magnetic resonance imaging (MRI) brain scans are increasingly used for investigation of other symptoms in elderly persons, therefore, MRI compatible devices should always be used.
Echocardiography
Echocardiography (ECHO) should be performed in syncope patients in whom a structural abnormality is suspected. The prevalence of structural cardiac abnormalities increases with age.81 The test is of most benefit in older patients with aortic stenosis98 and to evaluate ejection fraction. Cardiac arrhythmias are evident in up to 50 % of patients with an ejection fraction of less than 40 %.99
Ambulatory Blood Pressure Monitoring
Patterns of BP behaviour including post-prandial hypotension, hypotension after medication ingestion, orthostatic and exerciseinduced hypotension and SSH can be readily identified by this investigation. Modification of timing of meals and medications is guided by BP patterns.24,100
Exercise Stress Testing
Exercise stress testing is indicated to investigate cardiac disease and in patients who present with exercise-induced syncope.1 It is not always possible in older patients who may alternatively require angiography to investigate cardiac status.
Electrophysiological Study
Electrophysiological study is indicated in the older non-frail patient with syncope when a cardiac arrhythmia is suspected.24 Diagnosis is based on confirmation of an inducible arrhythmia or conduction disturbance.101 The benefit is dependent on pretest probability based on the presence of organic heart disease or an abnormal ECG.102
Electrophysiological study has the advantage of providing both diagnosis and treatment in the same session (i.e. transcatheter ablation).24 It is most effective for identification of sinus node dysfunction in the presence of significant sinus bradycardia of 50 bpm or less; prediction of impending high-degree atrioventricular (AV) block in patients with bifascicular block; inducible monomorphic VT (in patients with previous myocardial infarction [MI]) and inducible SVT with hypotension in patients with palpitations.24
Management of Cardiac Syncope
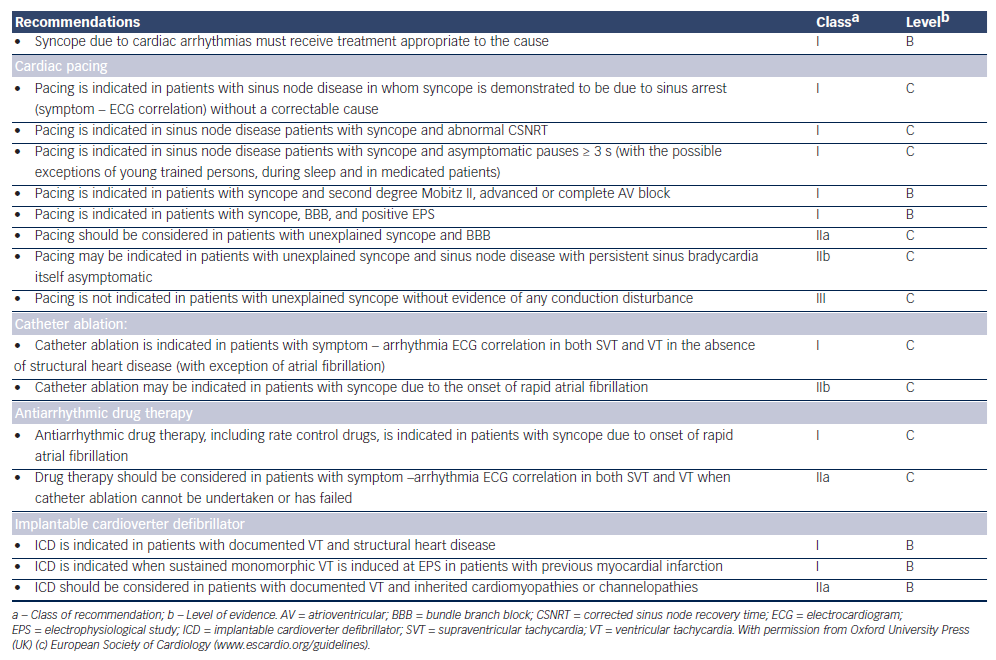
Management of cardiac syncope is dependent on specific cardiac diagnosis as outlined in Table 6.1
Challenges in the Older Patient
Frailty
As people are living longer, frailty and pre-frailty are more commonly encountered in clinical practice. Frailty is a reduction in the ability to respond to stressors and an increased vulnerability to adverse outcomes.103 There is no consensus on how best to operationalise or define frailty but two types of definitions have emerged as the most commonly used constructs: the Cumulative Burden Index as proposed where frailty is defined as an accumulation of health conditions and deficits, and the ‘Biological Syndrome Model’ as proposed by Fried:103 a person is deemed to be frail if they present with three or more of: poor grip strength, slow walking speed, low levels of physical activity, exhaustion and unintentional weight loss. Frailty is a predictor of falls, hospitalisation, disability and death.103
Unwitnessed Events in the Older Person
In the older adult a witness account may not be available for falls or syncopal events in up to 40 % of patients.16
Medications, Polypharmacy and Syncope
Polypharmacy is more common with advancing age. Some of the most frequently prescribed syncope-related medications used in combination are anti-hypertensives, anti-anginals, anti-histamines, anti-psychotics, tricyclic anti-depressants and diuretics. These cause bradycardia, QT interval prolongation, OH and VVS. Drug interactions can also cause syncope particularly in the older patient with multi-morbidity and polypharmacy.104 A temporal association between onset or change of medication and symptoms may be evident although progression of age-related physiological changes may cause syncope even with longstanding established medication use.24
TILDA reported an increased risk and frequency of syncope with use of tricyclic anti-depressants.105 The side effect most frequently reported is hypotension, but bradycardia and tachycardia have also been reported.106,107
Cognition
Cognitive impairment rises with age: 20 % of people over 80 years have established dementia,108 rising to 40 % over 90 years.109 Cognitive impairment is characterised by memory problems, attention difficulties and executive dysfunction – hence compliance with cardiac monitoring systems may be compromised.
Cognitive impairment is particularly high in older patients with CSH.55 Likewise, patients with some subtypes of dementia such as Lewy Body dementia55 and Alzheimer’s dementia have a higher prevalence of syncope, OH and CSH.108 Establishing a causal relationship between symptoms and arrhythmia or hypotension is particularly difficult in these patients given that the history is not reliable and events are often unwitnessed.3,6,110
There is emerging evidence that low BP may cause or exaggerate cognitive dysfunction,111 possibly because cerebral hypoperfusion is associated with cerebral damage via small vessel arteriosclerosis and cerebral amyloid angiopathy, as well as exaggerated white matter disease.112
Dual Diagnosis
In the older patient multiple causes of syncope may be present including cardiac (bradyarrhythmia, SVT tachyarrhythmias, ventricular tachyarrhythmias, long QT) and reflex syncope or autonomic impairment Table 3.22 Attribution of cause in the context of multiple abnormalities is not always possible, and treatment of all possible causes is recommended.
In one series of patients with syncope, mean age 66.5 years ± 18 years; 23 % had a dual diagnosis. The principal predictors of dual diagnosis were advanced age, treatment with alpha-receptor blockers and benzodiazepines. The most frequent dual diagnoses were OH and VVS: 2.8 % had a triple diagnosis, and these were the oldest old.28
Focal Neurology with Syncope
Transient ischaemic attacks or stroke and syncope are considered mutually exclusive presentations. However, one recent series reported that 5.7 % of syncope patients experienced focal neurological events at the time of syncope or pre-syncope. Awareness of this phenomenon is important to prevent misdiagnosis of stroke and inappropriate increase of anti-hypertensive medications, which would further exacerbate hypotensive symptoms.113,114
Conclusion/Summary
One of the greatest achievements of public health in the twentieth century has been the almost doubling of life expectancy in the Western world. Healthcare professionals are increasingly treating more old and very old patients. The prevalence of syncope rises with age and is challenging because of atypical presentation, overlap with falls and poor recall of events. Elders are less likely to have a prodrome, may have amnesia for loss of consciousness and unwitnessed events. Cardiac causes and dual pathology are more common and compliance with newer monitoring technologies is inadequate. Consequent morbidity and mortality is higher than in younger patients. A high index of suspicion for cardiovascular causes of falls and dual pathology will increase diagnosis and early targeted intervention.