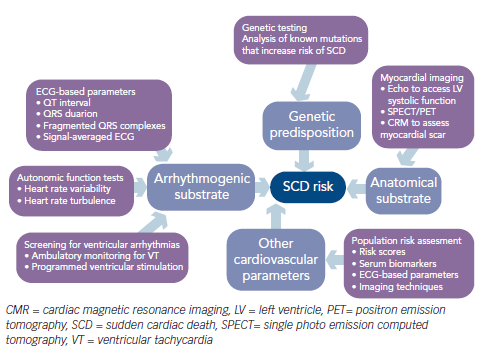
Sudden cardiac death (SCD) can be defined as unexpected death that occurs within one hour of the onset of symptoms or during sleep in a person who was previously stable. The mode of death, which may be due to an arrhythmic or non-arrhythmic cause, depends on the underlying cardiovascular abnormality (mechanical or electrical substrate). SCD remains a major public health problem worldwide and is estimated to account for 15–20 % of all deaths.1 Those deemed to be at highest risk of SCD may benefit from potentially life-saving treatment, such as insertion of an implantable-cardioverter defibrillator (ICD). However, such treatments are expensive and have their own associated risks, such as the risk of long-term infection and need for repeated surgeries and regular check-up. Hence, there is great interest and clinical need in improving methods for risk stratification of SCD to identify those at greatest risk.
Several factors make research into SCD risk stratification difficult and challenging. First, current guidelines and most international randomised studies on SCD have focused on specific high-risk groups – in particular, patients with reduced (below 30–35 %) left ventricular ejection fraction (LVEF) – with the aim of identifying those patients who would benefit most from an ICD. The latest European Society of Cardiology guidelines on ventricular arrhythmias and SCD recommend ICD therapy in patients with symptomatic heart failure (New York Heart Association [NYHA] class II–III) and LVEF ≤35 % after ≥3 months of optimal medical therapy who are expected to survive for at least 1 year with good functional status.2 Similarly, the American College of Cardiology and American Heart Association guidelines recommend ICD therapy in patients with LVEF ≤35 % due to prior myocardial infarction (MI), at least 40 days post MI, or non-ischaemic dilated cardiomyopathy and NYHA class II or III.3 Although low LVEF identifies patients at increased risk for cardiac arrest, the majority of sudden deaths occur in patients with LVEF greater than 30 %.4,5 Indeed, most cases of SCD occur in the general adult population, in people who may be relatively asymptomatic but have an underlying predisposition to SCD. Current guidelines on SCD risk stratification do not adequately cover this general population pool, as acknowledged in the latest European guidelines,2 yet from a population health perspective, this is the group that should be targeted if healthcare providers are to make any meaningful impact on lowering the incidence of SCD worldwide. Second, the mechanisms leading to SCD are complex and multifactorial, making the development of SCD risk scores and algorithms challenging. Even in those at highest risk of SCD, patient factors, comorbidities and underlying cardiovascular substrate abnormalities that predispose to SCD may change over time. Accurate methods for SCD risk stratification should take into account the changing and heterogeneous risk in each individual over time so that the risk is continually revisited and refined. In view of the wide and varying mechanisms and cardiovascular abnormalities underlying SCD, current methods for SCD risk stratification encompass a variety of modalities, which include imaging techniques to look for underlying mechanical or structural abnormalities, assessment for electrical substrate abnormalities and autonomic dysfunction and genetic analyses (see Figure 1).
This review will give an update of current methods and strategies for SCD risk stratification in high-risk individuals (namely patients with ischaemic and non-ischaemic dilated cardiomyopathy) and in the general public. Discussion of SCD in patients with inherited cardiomyopathies, primary arrhythmogenic diseases and genetic determinants of risk is beyond the scope of this review.
Risk Stratification in Patients with Ischaemic Cardiomyopathy
Imaging Techniques
The formation of myocardial scarring and left ventricle (LV) dysfunction following acute myocardial infarction (AMI) can result in areas of heterogeneous electrical conduction and abnormal electrical circuits within the diseased myocardium, which can predispose patients to the later development of ventricular tachycardia/ ventricular fibrillation (VT/VF) and SCD.6,7 Hence, imaging modalities demonstrating the presence of large areas of myocardial scarring or severe LV dysfunction may provide some indication of SCD risk. Measurement of LVEF is a crude indicator of the degree of myocardial damage and can be easily done using transthoracic echocardiography. The link between a low LVEF and risk of SCD has been well established.8 Consequently, LVEF was an important parameter measured in many of the early landmark randomised ICD trials and remains an integral component of current international guidelines on the indications for ICD implantation in patients following AMI for both primary and secondary prevention. However, a major limitation of the use of LVEF as a risk-stratification tool for determining which patients would benefit most from an ICD is that it is a good predictor of overall mortality but less accurate in predicting the development of VT/VF.9,10 More sophisticated imaging modalities for assessment of myocardial scarring have been found to be better predictors of the development of ventricular arrhythmias in patients with ischaemic cardiomyopathy. For example, strain imaging echocardiography appears to provide better prediction of VT/VF and SCD than LVEF alone, particularly in patients with LVEF > 35 %, in patients >40 days post AMI.11 Single photo emission computed tomography (SPECT) and positron emission tomography (PET) have also been shown to identify myocardial scarring and have a value in prediction of arrhythmic events in patients post AMI.12
The use of cardiac magnetic resonance (CMR) in assessing myocardial scar burden among AMI survivors and predicting mortality and arrhythmic events has been well explored and found to be of benefit by several investigators.13–16 The advantage of CMR over SPECT or PET imaging is that it has a much greater spatial resolution and is not dependent on vascular perfusion, so can also be used to identify scarring in non-ischaemic cardiomyopathies. Furthermore, CMR not only provides information on the overall scar burden and distribution, but also differentiates between different types of scarring, which provides further information on the underlying electrophysiological substrate abnormality. Quantification of the peri-infarct zone using contrast-enhanced CMR was shown to be an independent predictor of mortality following AMI in early studies.13 Other investigators have demonstrated that tissue heterogeneity in the peri-infarct zone, as detected by contrast-enhanced CMR, is likely to signify a pro-arrhythmic substrate and is one of the strongest predictors of ventricular arrhythmias and appropriate ICD therapies.14–16 Studies correlating myocardial scarring on CMR with invasive electrophysiological (EP) data from VT studies have shown that areas of heterogeneous scarring are more arrhythmogenic than areas of dense scarring and an independent predictor of VT/VF.17,18 More recently, investigators have shown that the extent of peri-infarct zone detected by CMR post AMI correlated with increased ventricular inducibility, even in patients with relatively preserved LVEF.19
ECG-based Parameters for Risk Assessment
Several ECG-based parameters, including QRS duration, fragmented ECG complexes and signal-averaged ECG (SAECG), have been widely studied in the context of prediction of ventricular arrhythmias in patients with ischaemic cardiomyopathy.20 Although a number of studies have shown some value in these tests, their overall positive predictive accuracy has been insufficient to allow them to be used solely as a risk-stratification tool.
The presence of fragmented QRS complexes (fQRS) on the routine 12-lead ECG has been described as a marker of abnormal ventricular depolarisation and demonstrated to be a predictor of mortality and sudden cardiac death.21,22 fQRS is a simple, inexpensive and easily accessible ECG sign that may be of value in determining the risk for SCD and guiding prophylactic ICD insertion in AMI survivors. In a recent meta-analysis of 12 studies involving 5009 patients, the presence of fQRS complexes was associated with all-cause mortality and SCD.23 Hence, fQRS complexes appear to be a useful marker of increased SCD risk. However, a greater understanding of the significance of this non-specific finding and future prospective, multicentre data is required before it can routinely be adopted into clinical practice.
The prognostic value of SAECG in predicting mortality among AMI survivors has been examined in multiple studies over the past few decades.24,25 The sensitivity of SAECG to predict arrhythmic events has been very variable from these studies, ranging from 15 % to 75 %, with follow-up of between 6 and 24 months. The main value of the SAECG appears to be its use in identifying low-risk patients in view of its high negative predictive value (over 90 %). However, its positive predictive accuracy is much lower, thus decreasing its usefulness as a single variable to identify high-risk patients.24 The coronary artery bypass grafting (CABG) Patch Trial was an important negative study in which SAECG appeared to be unhelpful in identifying a high-risk group of patients.10 With the increasing use of primary percutaneous coronary intervention (PCI) in the treatment of AMI, the prognostic value of the SAECG has become less clear. Bauer et al. performed SAECGs in 968 patients following AMI, 91 % of whom underwent PCI, and found that the presence of ventricular late potentials (VLPs) was not significantly associated with cardiac death or a serious arrhythmic event during a median follow up of 34 months.26 Ikeda et al. also found that VLPs had no significant prognostic role in predicting the primary outcome of death or resuscitated cardiac arrest when measured in 627 patients post AMI (82 % underwent PCI).27 The value of the SAECG in arrhythmic risk prediction among post-AMI survivors may be increased when it is used in combination with other tests to further refine risk in patients already deemed to be at higher risk, such as those with decreased LVEF. Gomes et al. demonstrated that the combination of an abnormal SAECG and LVEF<30 % in 1268 patients with coronary artery disease and nonsustained VT identified a particularly high-risk subset of patients that represented 21 % of the total population.28 In this group, 36 % and 44 % succumbed to arrhythmic and cardiac death, respectively.
Microvolt T-wave alternans (MTWA) has also been found to be a powerful predictor of life-threatening arrhythmias and SCD in patients post AMI, both with and without decreased LVEF.27,29,30 MTWA appears to be a better risk predictor when compared with SAECG31 and may be even more powerful when combined with LVEF and invasive electrophysiological (EP) testing.32 In a prospective multicentre study involving 575 patients, Chow et al. found that MTWA testing in patients with ischaemic heart disease and LVEF<30 % who already qualified for an ICD did not predict subsequent ventricular arrhythmic events, although MTWA non-negative patients (i.e. positive and indeterminate MTWA results) had significantly higher mortality compared with MTWA negative patients.33 The value of MTWA in risk stratification may actually be in deciding which patients are least likely to benefit from ICD insertion, as suggested by the ABCD (Alternans Before Cardioverter Defibillator) trial.34 This prospective, multicentre study was the first to use MTWA to guide prophylactic ICD insertion. The investigators demonstrated that MTWA achieved one-year positive and negative predictive values of 9 % and 95 %, respectively, and that its use in risk stratification was comparable to invasive EP study at one year and complementary when applied in combination.
Autonomic Function Tests
Autonomic function tests, such as heart rate variability (HRV) and heart rate turbulence (HRT), have also been extensively studied because autonomic dysfunction can increase the risk of ventricular arrhythmias, especially in patients post AMI. Evidence suggests that decreased HRV is associated with increased ventricular arrhythmias and mortality.35,36 In the Multicenter Postinfarction Study (MPS), which involved 808 patients, a strong correlation was found between reduced HRV and total mortality following AMI.37 However, HRV does not appear to fare as well as other markers of autonomic dysfunction when directly compared. In the Autonomic Tone and Reflexes After Myocardial Infarction (ATRAMI) study, which involved 1284 patients with a recent (<28 days) MI, baroreceptor sensitivity (calculated from measuring the rate–pressure response to intravenous phenylephrine) was a better predictor of mortality, particularly in patients with LVEF<35 %.36
Several large-scale prospective studies have provided strong evidence that HRT is a powerful independent predictor of risk following AMI.38–40 In the REFINE study, autonomic function tests, including measurements of HRT, were conducted in 322 patients with LVEF <50 % post AMI. The investigators found that these tests could reliably identify those at high risk of serious cardiac events.39 Another prospective6 study involving 2343 survivors of AMI found that the combination of HRT and deceleration capacity could be used together to identify a high-risk group equivalent in size and mortality to patients with LVEF<30 %.40
Role of Invasive Electrophysiological Testing
Early studies on the use of invasive EP testing to risk-stratify patients at increased risk of malignant ventricular arrhythmias were performed in AMI survivors – reports from these studies were conflicting, with nearly half of all studies finding that the inducibility of sustained VT was unhelpful in predicting later mortality or arrhythmic events.41,42 The apparent confusion in the literature is probably related to differences in patient population, stimulation protocols and time intervals between AMI and EP testing.41,42 In addition to the invasive nature of the test and need for specialist equipment and personnel, another limitation to the routine use of EP testing in risk prediction includes the wide range of reported sensitivities (between 28 % and 80 %). The future role of this invasive test in risk prediction may lie in its combined use with other non-invasive tests, such as MTWA and HRV, to further refine the selection of potential ICD recipients.34,43,44
Risk Stratification in Patients with Non-ischaemic Dilated Cardiomyopathy
Risk stratification of SCD in patients with non-ischaemic dilated cardiomyopathy (NIDCM) has been less studied than in patients with ischaemic heart disease and depressed LVEF. There is as great a need for accurate and reliable risk stratification in NIDCM patients because they tend to be younger and have a better prognosis and therefore may receive less overall benefit from an ICD than patients with ischaemic cardiomyopathy.45 The pathophysiology of VT/VF in patients with NIDCM is less understood and is likely to be due to a variety of mechanisms, including myocardial fibrosis, left ventricular dilatation and autonomic dysfunction. Consequently, despite the plethora of tests available for SCD risk stratification, there is currently no definite test or recommendation for this population other than using the LVEF, which is a crude estimate of VT/VF risk. Unlike the potential value of autonomic function tests in SCD risk stratification in patients post AMI, these tests do not appear to be useful in patients with NIDCM. In a recent study of 60 patients with NIDCM and LVEF≤50%, Pezawas et al. demonstrated that a variety of non-invasive tests (including pharmacological baroreflex testing, short-term spectral analysis of HRV, long-term time domain analysis, exercise MTWA, SAECG, and corrected QT-time) could not reliably identify patients at risk of fatal ventricular arrhythmias.46 In a meta-analysis of 45 studies involving non-invasive tests to predict the risk of arrhythmic events in 6088 patients with NIDCM, Goldberger et al. found that fragmented QRS complexes and T-wave alternans (TWA) had the best odds ratios, whereas none of the autonomic tests (HRV, HRT and baroreflex sensitivity) were significant predictors.47 In view of the heterogeneous nature of NIDCM and the multiple mechanisms that underlie the pathophysiology of VT/VF, a different strategy and combination of tests is probably required to optimise risk stratification in this patient population.
Risk Stratification in the General Population
From a population perspective, reliable identification of people in the general public at highest risk of malignant ventricular arrhythmias would provide the greatest impact on decreasing the incidence of SCD. Since a major cause of SCD is in the context of an acute coronary syndrome or myocardial infarction, identification of those at risk of an acute atherosclerotic event and subsequent VT/VF may be a useful avenue to pursue. Although risk stratification for coronary artery disease is well established in clinical practice, there are currently no accurate methods available to identify individuals at risk of plaque rupture and the development of VT/VF. Epidemiological studies on certain serum biomarkers, such as N-Terminal pro-B type natriuretic peptide, non-esterified fatty acids, interleukin-6 and circulating antibodies, appear to show some potential in helping to identify those in the general public at risk of acute plaque rupture and sudden VT/VF,48–51 although more data is required before such biomarkers can be used clinically. Advances in cardiac CT technology have allowed the characterisation of atherosclerotic plaque features that can provide additional information on the risk of plaque rupture and subsequent SCD.52–54 However, this imaging modality would not be suitable for mass screening on a population level. Other imaging modalities, such as echocardiography, may have a role to play in SCD risk assessment in the general population. For example, echocardiographic measurements of left ventricular mass and diameter have been shown to be associated with an increased risk of sudden death among the general public.55,56
A more practical method to assess SCD risk in the general population is to use ECG-based parameters and large-scale screening. A Finnish cohort of over 10,000 middle-aged subjects has helped provide some of the most useful data on ECG-based risk of SCD to date.57–60 In this cohort, QRS prolongation of ≥110 ms, intraventricular conduction delay (but not bundle branch block), and early repolarisation of at least 0.2 mV, were all independent predictors of arrhythmic death. However, the low prevalence of each of these markers may limit their utility in large-scale screening.
The clinical significance of early repolarisation on the surface ECG has gained increasing interest following two seminal studies demonstrating a link between early repolarisation syndrome (ERS) and idiopathic VF/sudden death.60,61 Investigators have since compared ERS in cardiac arrest survivors with preserved ejection fraction,62 in families with sudden arrhythmic death syndrome63 and other families with an early repolarisation pattern on the ECG64 and in Asian populations.65 ERS in the inferior leads, especially in cases without other QRS complex abnormalities, appears to predict the occurrence of VT/VF but not non-arrhythmic cardiac events, suggesting that early repolarisation is a specific sign of increased vulnerability to ventricular arrhythmias.66 Programmed ventricular stimulation was not found to enhance risk stratification in patients with ERS, even among those at highest risk who survived a cardiac arrest.67 Despite the increasing body of evidence in this area, controversy still remains on the exact clinical significance, implications and optimal management in patients with ERS on the surface ECG.68,69
Other novel ECG-based parameters may also be potentially useful in population screening for SCD. In a recent study of 14,024 participants without left ventricular hypertrophy in the Atherosclerosis Risk in Communities (ARIC) study, beat-to-beat spatiotemporal variability in the T-vector on standard 12-lead ECGs was found to be associated with SCD over a median follow-up of 14 years.70 In another large, retrospective population-based cohort study using the ARIC database, ECG-deep terminal negativity of the P-wave in V1 was also found to be associated with an increased risk of SCD.71
Conclusion
It is clear that current methods for SCD risk stratification are inadequate, both in high-risk groups such as patients with reduced LVEF post AMI, patients with NIDCM and among the general public. Several novel risk-stratification techniques, including imaging, ECGbased methods and serum biomarkers, have shown considerable promise in refining SCD risk on top of conventional methods. However, such data has been derived from single-centre studies or studies involving a small number of centres, rather than large international randomised clinical trials. Hence, their clinical utility, effect on outcomes and cost-effectiveness require further evaluation and, consequently, these novel risk-stratification techniques have not yet been incorporated into current international guidelines on ICD therapy. Different approaches may be required for risk stratification of different populations. In high-risk groups, a combination of risk-stratification tests involving imaging and ECG-based techniques, in addition to conventional transthoracic echo to assess LVEF, is likely to be needed. Specific combinations may be required for patients with ischaemic and non-ischaemic cardiomyopathy, which will need to be validated prospectively. Repeat risk assessments may be appropriate in patients who are not deemed to be at high risk at baseline as the risk may change over time; hence, non-invasive tests may be more practical than invasive investigations such as EP testing. The approach to SCD risk stratification and reduction of risk in the general public will require a different angle because it is not possible or economically viable to perform sophisticated and expensive tests on a population level. The future in this area will probably lie in the use of improved clinical risk scores, possibly in combination with simple, inexpensive tests (ECGbased or serum biomarkers). These risk-stratification methods may be applied to certain subsets of the public who may be at increased risk, e.g. in males above the age of 40 and post-menopausal women, or those with other cardiovascular risk factors such as hypertension or diabetes. As it would not be practical to perform conventional randomised‚ controlled trials on a population level, validation of such an approach would require international epidemiological studies and good registry data.