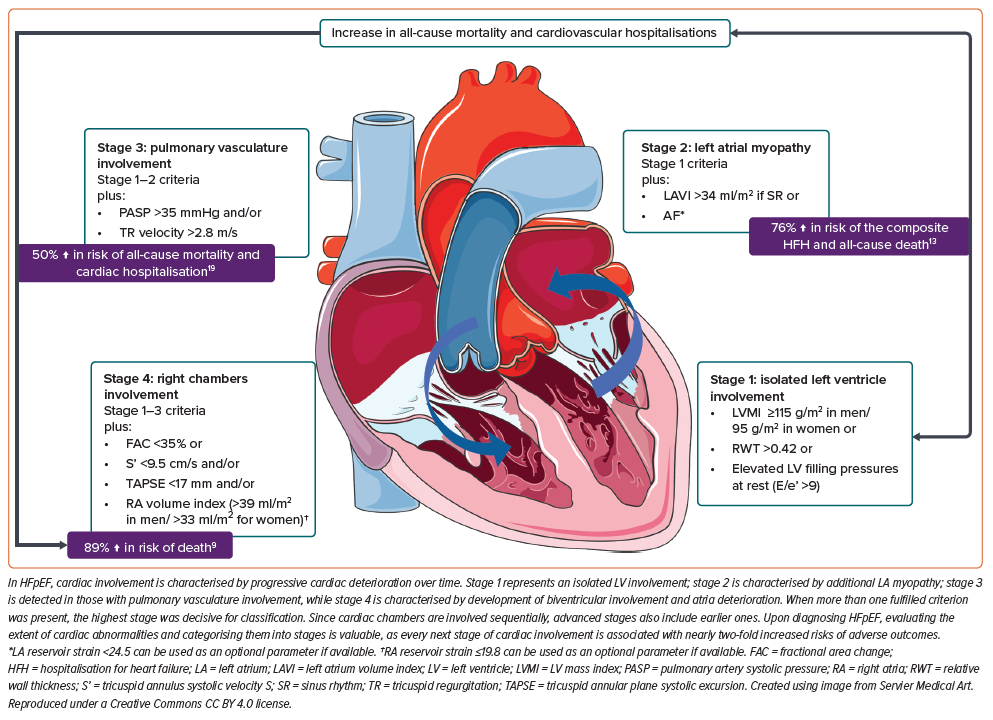
Heart failure with preserved ejection fraction (HFpEF) is a major global public health concern due to increasing incidence and prevalence, poor prognosis and limited availability of disease-modifying therapy. The management of HFpEF and the development of novel therapies is complicated due to the heterogeneous nature of the disease, which presents with multiple clinical phenotypes. Each is characterised by a unique combination of cardiac and non-cardiac comorbidities such as hypertension, obesity, type 2 diabetes, chronic kidney disease, chronic obstructive pulmonary disease and others. Numerous attempts have been made to phenotype HFpEF, but we still lack a clinically and/or prognostically relevant approach.1–3 Looking beyond the phenotypes, HFpEF is considered a systemic condition in which the prevalence of cardiovascular, metabolic, pulmonary and renal conditions determine the extent of cardiac involvement. Progressive cardiac deterioration in HFpEF appears to be associated with a worse prognosis. However, no attempt has been made to classify the extent of cardiac involvement in HFpEF. Here, we discuss emerging data suggesting that a staging classification for HFpEF focusing on the deterioration of cardiac chambers could be of prognostic value and become a reliable tool for clinicians and researchers (Figure 1).
Diagnosis of Heart Failure with Preserved Ejection Fraction
International heart failure (HF) guidelines state that the diagnosis of HFpEF is established when individuals exhibit symptoms and signs of HF and have a left ventricular ejection fraction (LVEF) ≥50%. There must also be evidence of spontaneous or provokable elevation in left ventricular (LV) filling pressures. This evidence can include elevated levels of natriuretic peptides and invasive and non-invasive haemodynamic measurements. Objective signs of structural and functional cardiac abnormalities consistent with LV diastolic dysfunction or elevated LV filling pressures are also essential. These abnormalities may include indices such as LV mass index, relative wall thickness (RWT), left atrium (LA) volume index (LAVI), E/e’ ratio at rest, pulmonary artery (PA) systolic pressure, and tricuspid regurgitation (TR) velocity at rest.4,5
Although the above-mentioned cardiac abnormalities consistent with either LV, LA or PA involvement are a part of the HFpEF diagnosis, not all patients have these disorders at the time of first presentation. Increasing evidence suggests that the extent of cardiac involvement will differ in HFpEF patients at any specific time. LA, pulmonary vasculature and right ventricle (RV) involvement can be found in 67%, 46% and 50% of patients, respectively.6–9 The pathophysiology of HFpEF progression suggests that the cardiac chambers become involved in a sequential manner.
Pathophysiology
HF mainly develops due to the following mechanisms: increased pressure, volume overload or direct myocardial injury, all of which may coexist. The presence of cardiovascular, metabolic, pulmonary and renal conditions leads to the activation of key underlying factors driving the development of HFpEF, including systemic low-grade inflammation and metabolic disturbances that ultimately affect the myocardium, cardiomyocytes and interstitial space, and vessels, at varying degrees.10 Intrinsic cardiac dysfunction results in elevated left ventricular end-diastolic pressure or left ventricular filling pressure (LVFP) – a hallmark and driver of HFpEF.
Progressive myocardial changes and sustained pressure overload of the LA lead to LA dilatation and AF, eventually resulting in involvement of pulmonary circulation in the form of secondary pulmonary hypertension. Prolonged haemodynamic impairment causes the development of TR and, finally, leads to RV dysfunction (RVD) and biventricular failure. Therefore, in pressure overload, the sequential involvement of heart chambers is likely to occur in HFpEF subjects and reflects the natural consequences of progressive damage. Of note, some patients with HFpEF may develop atrial functional mitral regurgitation (MR), therefore experiencing a volume overload and pressure overload.11
Isolated Left Ventricle Involvement
All HFpEF patients exhibit involvement of the left ventricle (LV). LV structural and functional abnormalities are characterised by LV hypertrophy, defined as an increased myocardial mass index and RWT, and diastolic dysfunction resulting in reduced LV myocardium compliance, elevated LVFP and decreased cardiac output.
Remarkably, LV involvement can also be classified into several types. In the study from the Asian HF registry, which included 1,765 patients with HFpEF, five echocardiographic clusters of HFpEF were identified and validated:
- Normal LV (25%): normal structure despite increased LVFP.
- Restrictive (26%): small cavities, concentric hypertrophy, small stroke volume.
- Hypertrophic (25%): concentric hypertrophy.
- High output (10%): greatest stroke volume.
- Atrial dominant (10%): most LA myopathy.
- Other (4%).
No statistical differences were found in survival rate over 2 years across the five clusters.12 Previous studies noted that about 26% of HFpEF patients with a diagnosis confirmed by invasive haemodynamic exercise testing had isolated LV deterioration.13 Whether HFpEF subjects with isolated LV involvement have a better prognosis than those with concomitant LA or RV involvement remains to be identified in studies which make direct comparisons of these subgroups.
Left Atrial Involvement
LA myopathy is defined as LA enlargement or impaired LA function. LA enlargement is characterised by LAVI>34 ml/m2 or >40 ml/m2 in patients with concomitant AF. LA function is assessed by echocardiography with strain measuring either a peak atrial longitudinal strain (PALS) or LA reservoir function with a cut-off >24% to define normal function.14 Studies have demonstrated an association between rising LVFP and the emergence of LA myopathy, laying the foundation for categorising the extent of cardiac damage in HFpEF patients. However, it is also crucial to acknowledge and address the role of primary atrial myopathy, which can contribute to HFpEF.15,16
A prevalence of LA involvement in HFpEF of 67% has been reported.7 In a more recent study by Omote et al. involving 467 HFpEF patients who underwent invasive exercise testing, LA myopathy was found in ~70% of individuals (31% had isolated LA and 39% had both LA and RA involvement).13 The atria remained unaffected in 26% of patients.13
Previous studies showed that LA myopathy in HFpEF was associated with new onset AF, AF progression to a permanent stage, and a higher rate of cardiovascular hospitalisation or death from any cause.6,7 Moreover, LA dysfunction was associated with increased pulmonary vascular resistance, decreased peak oxygen consumption and RV dysfunction, proving sequential patterns in disease progression.7,17
Pulmonary Vasculature and Right Chamber Involvement
The range of prevalence of pulmonary hypertension (PH) in HFpEF is 23–52.5%.18 Elevated pulmonary artery pressure in HFpEF may develop passively due to increased LA pressure. However, PH could also result from pulmonary vasculature vasoconstriction and remodelling occurring in settings of chronically elevated pulmonary venous filling pressures, resulting in the formation of pre-capillary PH in some patients.19 The presence of PH in HFpEF is associated with a high rate of 1- and 5-year mortality (23.6% and 48.2%) and hospitalisations for cardiac causes (28.1% and 47.4%).19
Pulmonary vascular disease in HFpEF is associated with an impairment of RV function.20 RV dysfunction is defined as reduced RV fractional area change, tricuspid annular plane systolic excursion (TAPSE) or tricuspid annular systolic velocity (RV S’). It was reported in 33–50% of HFpEF patients and has load-dependent and load-independent mechanisms.9,21 Patients with RV dysfunction had severe symptoms and a higher comorbidity burden.8 Moreover, RV dysfunction was the strongest single predictor of mortality, exceeding PH severity, comorbidities, and measures of left heart structure and function. Moreover, RV deteriorated over time.8 HFpEF patients who developed incident RV dysfunction had a nearly two-fold increased risk of death.8
The data on RA myopathy are limited. It has been suggested that RA myopathy should be defined as volume index >39 ml/m2 in men and >33 ml/m2 in women and/or RA reservoir strain ≤19.8% for both men and women.13 Individuals with HFpEF and biatrial deterioration had a higher burden of AF, poorer LV systolic and diastolic function, more severe pulmonary vascular disease, TR, RV dysfunction and an 84% higher risk of hospitalisation for HF or death when compared to those with isolated LA myopathy at 2.9 years follow-up.13
These findings underscore a gradual expansion of cardiac involvement over time, with elevated rates of adverse outcomes accompanying the progressive engagement of each cardiac chamber. This emphasises the necessity for staging of cardiac deterioration in HFpEF patients.
Staging of Cardiac Involvement in Patients with Various Cardiac Conditions
The concept of HF staging according to the extent of cardiac involvement identified by transthoracic echocardiography (TTE) was first proposed by Généreux et al. for patients with aortic stenosis (AS) and included five stages:22
- Stage 0: no extra-aortic valve damage.
- Stage 1: LV damage, defined by the presence of LV hypertrophy (LVMI >95 g/m2 in women and >115 g/m2 in men), and/or elevated LVFP (E/e’ ratio >14), and/or mild LV systolic dysfunction (LVEF <60%).
- Stage 2: LA and/or mitral valve deterioration defined by LA enlargement (LAVI >34 ml/m2), and/or AF, and/or moderate MR.
- Stage 3: pulmonary vasculature and/or tricuspid valve damage defined by the presence of systolic pulmonary hypertension (sPAP >60 mmHg) and/or the presence of moderate or greater TR.
- Stage 4: RV damage defined by RV systolic dysfunction determined by visual examination and quantitative assessment using tricuspid annulus systolic velocity S’ <9.5 cm/s and/or TAPSE <17 mm.
The staging concept demonstrated significant prognostic relevance. The proportion of 1-year all-cause death was the lowest in those with no extra-aortic valve cardiac damage (stage 0) at about 4.4% and increased progressively across the stages. Individuals with isolated LV involvement (stage 1) and those with additional LA deterioration (stage 2) demonstrated almost comparable rates of all-cause death, accounting for 9.2% and 14.4%, respectively.17 Patients presenting with pulmonary circulation involvement or manifest RVD had the highest rates of 1-year all-cause death at 21.3% and 24.5%, respectively. Later, this approach was adopted for individuals with MR.23
Considering the crucial role of progressive intrinsic cardiac dysfunction and elevated LVEP in the progression of HFpEF, as well as a trajectory of cardiac deterioration demonstrating a subsequent involvement of cardiac chambers with time, the staging detailed above can be used for HFpEF. The prognostic significance of every cardiac chamber involved can explain the rationale for this classification.
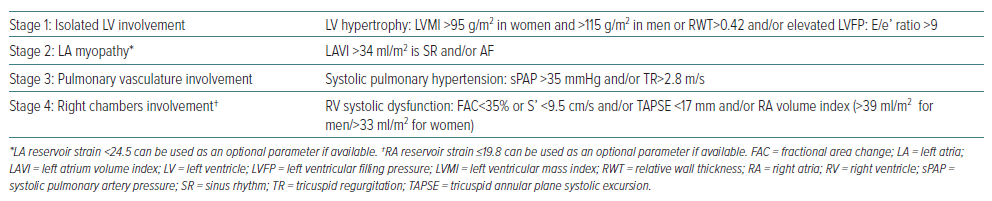
Staging of Cardiac Chamber Involvement in Heart Failure with Preserved Ejection Fraction
Before the staging classification proposed by Généreux et al. can be implemented for HFpEF patients, it must be adapted. We propose some modifications employing parameters of cardiac structural and/or functional abnormalities consistent with HFpEF as defined by the 2021 European Society of Cardiology guidelines for the diagnosis and treatment of acute and chronic heart failure and findings from relevant studies.4,13,14 First, while stage 0 (no extra-aortic valve cardiac damage) is relevant for AS individuals, it is not applicable for those with HFpEF, who have LV involvement by definition and so this stage should be omitted and cardiac deterioration in HFpEF should be categorised into four stages (Table 1).
Other modifications include changes to the cut-off for E/e’ ratio to >9 and adding RWT>0.42 as an additional measure of LV hypertrophy in stage 1, changes to the cut-off for sPAP to >35 mmHg in stage 3 with the addition of fractional area change with a cut-off <35% to identify RV systolic dysfunction.4,21 We also suggest using the parameters of LA and RA reservoir strain as optimal measures of atrial dysfunction when comprehensive TTE is available.
When more than one fulfilled criterion was present, the highest stage would be used for classification. Since cardiac chambers are involved sequentially, advanced stages also include earlier ones.
Upon diagnosing HFpEF, evaluating the extent of cardiac abnormalities and categorising them into stages is valuable. However, the staging classification based on cardiac involvement should be tested further and validated (Figure 1).
Future Perspectives and Implications of a Staging Classification for HFpEF
The proposed staging classification of HFpEF requires additional investigation and validation in HFpEF patients. Although it was developed by accumulating evidence from individual studies that differed in study design and sample size, the data are consistent across the studies regarding the prognostic significance of the involvement of every cardiac chamber, which suggests the proposed approaches can be used to assess a prognosis.
The staging concept has the potential to guide a therapeutic strategy for people with HFpEF. A promising summary of this perspective was given by Kozaily et al. in a review of the management of pulmonary hypertension in the context of HFpEF, discussing a therapeutic approach to isolated post-capillary PH (IpcPH) and combined pre- and post-capillary pulmonary hypertension (CpcPH) in HFpEF.24 The authors concluded that both sub-groups of patients should benefit from loop diuretics, sodium–glucose cotransporter-2 inhibitors, exercise and weight loss; may benefit from angiotensin receptor/neprilysin inhibitors and mineralocorticoid receptor antagonists; and would not benefit from endothelin-receptor antagonists and prostacyclin analogues.24 Of note, phosphodiesterase inhibitors, such as soluble guanylate cyclase, had no effect on IpcPH HFpEF, but hypothetically, they could have a benefit for those with CpcPH and further studies with appropriately selected patients are required. We believe that the proposed staging classification might be helpful in this area.
A prospectively validated HFpEF staging classification could serve as a road map for future clinical trials to identify effective treatment and preventive strategies to mitigate disease progression at each stage.
In HFpEF, the involvement of cardiac chambers develops sequentially and can be classified into four stages using transpacific electrocardiography. An increasing amount of evidence suggests that extension of cardiac deterioration might have a prognosis significance. If true, the staging approach could guide future clinical trials on HFpEF pharmacotherapy.