Cardiovascular disease (CVD) is the leading cause of death among women in the US and globally.1 Although the overall death rates from CVD have decreased in recent years, rates of acute MI (AMI) and CVD mortality have actually been increasing among young US women aged <65 years.2–4 One analysis found that the prevalence of AMI among young women aged 35–54 years increased from 21% in 1995 to 31% in 2004; in comparison, the prevalence of AMI in men over the same time period increased from 30% to 33%.4 Between 2011 and 2017, middle-aged women, defined as those aged 45–64 years, had a 7% increase in death rates, compared with a 3% increase among men during this time period.3 Women are at risk of a broad spectrum of CVDs, including, but not limited to, CHD including AMI, stroke and heart failure (HF).1
Women typically present with atherosclerotic CVD (ASCVD) approximately a decade later than men during their postmenopausal period due to decreases in oestrogen and a loss of its protective effects.5,6 Loss of the protective effects of oestrogen leads to worsening of traditional risk factors: weight gain, insulin resistance and higher blood pressures.7 Thus, older women presenting with CVD are also more likely to present with comorbid conditions such as diabetes and hypertension.5,8 Available evidence suggests that some traditional risk factors, such as diabetes and smoking, confer a greater relative risk of ASCVD in women than men.9,10
In addition, women are at risk of CVD due to female-specific risk factors (e.g. adverse pregnancy outcomes, polycystic ovary syndrome and premature menopause) or factors that are more prevalent in women (e.g. autoimmune disorders, including lupus and rheumatoid arthritis, and radiation or chemotherapies for breast cancer).7,11,12 These added risks are often unaccounted for, leading to underestimation of cardiovascular risk and the under-treatment of women.13 Recognition of the multitude of factors that predispose women to CVD can be instrumental in addressing the burden of disease. However, current risk scores do not take into account such comorbidities, although some guidelines do consider them ‘risk-enhancing’ factors.13,14
Both primary prevention (before initial presentation of disease) and secondary prevention (after initial presentation of disease) strategies are crucial to decrease the burden of CVD for women. Primary prevention of CVD in women has been covered in other recent reviews.10,15 Given space limitations, this review will focus primarily on secondary prevention strategies and guidelines. This review serves to highlight multidisciplinary guidelines for women across a broad spectrum of CVD, including known atherosclerotic disease, HF and post-MI, post-cardiac catheterisation and post-coronary artery bypass grafting (CABG) surgery.
Outcomes After MI and CABG
Women across all age groups are susceptible to poor outcomes from CVD. Older women are more likely to experience mortality after a percutaneous intervention for an ST-elevation MI (STEMI), but not after non-ST-elevation MI (NSTEMI).8 Younger women <55 years who experience AMI are less likely to receive guideline-directed medical therapy (GDMT), have a greater likelihood of readmission within 1 year, have worse self-reported recovery outcomes and have higher rates of all-cause mortality.16–19 Women have also been shown to be less likely to undergo CABG than men and to have worse outcomes after CABG.20 This discrepancy is thought to be due to women presenting at older ages, with more comorbidities and with later-stage CVD. Despite women being at such high risk for secondary cardiovascular events, most cardiovascular trials to date have been predominantly male, with females being under-represented in trials for CHD, acute coronary syndrome (ACS) and HF relative to their disease burden in the population.21–25 This limits the knowledge base of the efficacy and safety of cardiovascular preventive interventions and limits generalisability of trial results into clinical practice. Even so, available evidence does indicate that women derive a nearly similar benefit from existing secondary prevention pharmacological treatment modalities such as statins, ezetimibe, proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors and icosapsent ethyl as men, as discussed further below.26–31
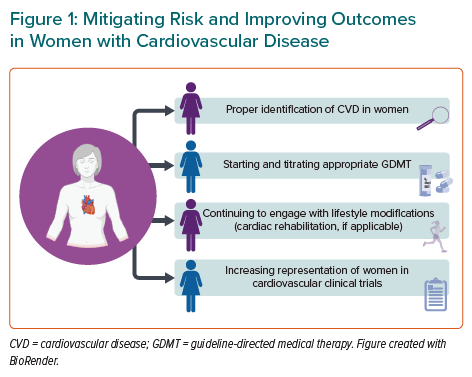
Thus, more attention for secondary CVD prevention in women is sorely needed, with additional attention to mental health and social determinants of health. Figure 1 outlines some strategies for mitigating risk and improving outcomes after CVD in women. It should be further noted that there are knowledge gaps on how to reduce cardiovascular risk among transwomen and transmen, which warrants further study.
Atypical Presentations and Obstacles to Treatment
Difficulty in treating women with GDMT for CVD begins with the initial diagnosis. Compared with men, women describe overall milder symptoms and are more likely to describe weakness, shortness of breath and fatigue as opposed to squeezing pressure, heart burn and palpitations.6 Women are also more likely to report pain in the chest, back or jaw without chest pain.6 In the setting of AMI, women do experience chest discomfort to a similar degree as men (~90% of cases), but are more likely to report three or more additional symptoms that may distract patients and their clinicians from initial recognition of the true diagnosis.32,33 As a result of these different presentations and overall lower perceived risk of CVD, women often have delayed diagnoses and are less likely to get urgent revascularisation of their MI.34–36
One study examining revascularisation times of young individuals (<55 years of age) presenting with AMI found that 35% of women presented more than 6 hours after any initial symptoms, compared with 23% of men.36 In that study, women had 1.72-fold (95% CI [1.28–2.33]) the odds of exceeding the reperfusion time goal, and 2.31-fold (95% CI [1.32–4.06]) the odds of not receiving reperfusion at all.36 After the cardiovascular event, despite the strong evidence behind secondary prevention guidelines, women are not placed on appropriate GDMT and have worse patient-reported outcomes.37,38 For example, women are less likely to be treated with statin or aspirin therapy and have controlled hypertension, and, overall, are less likely to be linked to appropriate cardiac rehabilitation (CR) programmes.37,39 Disparities in access to care for women extend throughout all ages and stages of CVD. Previous reviews have demonstrated that minimising modifiable risk factors at every aspect of care from diagnosis to treatment can help close the gap and improve outcomes.40
Sex Differences in Medication Side-effects
Women often report greater overall medication side-effects than men, which often leads patients or their clinicians to stop medications or decrease dosages to a more tolerable side-effect profile.41,42 Side-effect profiles of cardiovascular medications for women are variable and stem from differences in gastrointestinal absorption, body composition, metabolic consumption and kidney excretion. For example, one large US survey found that women were 28% more likely to have new or worsening muscle symptoms with statin therapy (adjusted OR 1.28; 95% CI [1.16, 1.42]) and 48% more likely to discontinue their statin therapy due to muscle symptoms (adjusted OR 1.48; 95% CI [1.25–1.75]) than men.42 This is worrisome in light of the substantial benefit that women derive from statin therapy.27 As another example, women have greater hospitalisations as a result of side-effects from torsemide, with higher circulating plasma concentrations of the drug.43 There are certainly many more examples.
In such cases of intolerability, often a lower dose of medication can still provide benefit without the added risk. In situations where a reduced dosage is used due to side-effects, clinicians should consider titrating up to the maximum tolerated dose to make best use of benefits. Acknowledging and educating both clinicians and patients on the side-effect profile of cardiovascular medications is crucial in mitigating the risk of such medications and helping tailor the appropriate medication regimen. These discrepancies further highlight the need for increased representation of women in pharmacological cardiovascular trials.
MINOCA, INCOCA and Coronary Microvascular Dysfunction
Women presenting with AMI are also twice as likely to present with MI with non-obstructive coronary arteries (MINOCA) over obstructive coronary artery disease (CAD).44,45 Women who present with MINOCA more often present with NSTEMI as a result of plaque erosion or rupture, coronary embolus or thrombosis, microvascular dysfunction, coronary artery dissection or spasm.45 Plaque erosions are thought to evolve from endothelial apoptosis, whereas plaque rupture is the result of inflammation. Both are associated with some coronary evidence of atherosclerotic burden. Women who are predisposed to a hypercoagulable state from antiphospholipid syndrome or inherited thrombophilia are at risk for MINOCA from coronary thrombosis and embolism of the microcirculatory system.
Another cause of MINOCA results from spontaneous coronary artery dissection (SCAD), where there is separation of the layers of the epicardial coronary artery wall from an intimal tear or an intramural haemorrhage, which results in decreased arterial flow. SCAD may account for up to one-third of MIs among young women aged <50 years. SCAD is managed differently than other atherosclerotic-type MIs and thus is not discussed here; however, the topic has been comprehensively reviewed elsewhere.46 Although the underlying pathophysiology of each of the above causes of MINOCA is distinct, all result in decreased forward flow and poor perfusion of myocardial tissue, thus leading to ischaemia and infarct.
A combination of coronary optical coherence tomography (OCT) or intravascular ultrasound (IVUS) plus cardiac MRI may help elucidate the aetiology of MINOCA in the vast majority of women.45,47 IVUS/OCT has been shown to be useful in detecting plaque disruption, coronary embolus or thrombus and SCAD.48 Despite overall better outcomes for individuals with MINOCA than those with complete occlusive disease, young women have higher mortality and adverse events from MINOCA than young men. The cause of this differential in mortality has not been studied, but is thought to be due, in part, to failure of placing women on appropriate goal-directed therapy.48 Preliminary studies from the SWEDEHEART registry indicate beneficial effects of statins, angiotensin-converting enzyme inhibitors (ACEI) or angiotensin II receptor blockers (ARB), and β-blockers for women with MINOCA.49
Whereas MINOCA reflects AMI, women can also have stable angina and/or evidence of coronary ischaemia with non-obstructive coronary arteries (INOCA).50,51 Among individuals with stable angina and evidence of ischaemia, one study found that 65% of women had no obstructive CAD, compared with 32% of men.50 Among individuals with moderate to severe ischaemia discovered on stress testing who were screened for eligibility to participate in the ISCHEMIA trial, 66% of those found to have no obstructive disease (the population enrolled in CIAO-ISCHEMIA registry) were female, compared with 26% of those with obstructive CAD (the population enrolled in the main ISCHEMIA trial).52 The frequency of angina symptoms and the amount of abnormalities seen on stress echocardiography testing (i.e. inducible wall motion abnormalities) were actually surprisingly similar between patients with and without obstructive CAD (i.e. patients enrolled in the ISCHEMIA trial versus those enrolled in the CIAO-ISCHEMIA registry). Furthermore, the degree of ischaemia on stress testing was not significantly correlated with symptom burden (i.e. angina). One may have assumed that the more ischaemia, the more symptoms and that if one could reduce ischaemia, that would reduce symptoms, but that is not the case. Ischaemia and angina were found to be not that well correlated.52
Nevertheless, INOCA in women is not benign and is associated with an elevated 5-year risk of major adverse cardiovascular events (MACE) compared with women without angina.53 Non-obstructive plaque is prognostic of MACE risk in women and should not be ignored when detected on imaging evaluation, and plaques with high-risk features may confer even greater relative risk in women than in men.54–57
INOCA can be the result of coronary microvascular dysfunction, vasospastic angina (VSA) or a combination of both, but is often underdiagnosed and undertreated and often has a poor prognosis.58 INOCA from microvascular dysfunction is defined as typical chest pain, non-obstructive coronary arteries and impaired flow specifically seen through one of the following: poor coronary flow reserve (CFR); spasm during provocative testing; or decreased coronary blood flow.59
Both anatomical and functional testing are frequently used for the work-up of women with suspected angina, and provide complementary information. Anatomical approaches (e.g. invasive coronary angiography or coronary CT angiography) rule out obstructive disease and can identify non-obstructive atherosclerotic plaque that warrants implementation of preventive therapies. Functional testing is used to evaluate for ischaemia. Ischaemia can be first identified through a number of non-invasive methods, including PET scans or dobutamine stress echocardiography. PET can be useful in detecting microvascular dysfunction and thus CFR, which is a strong indicator of prognosis.60 However, nuclear PET scans cannot identify coronary vasomotor disorders, which are common causes of INOCA in women.58
Elucidating coronary artery spasm requires acetylcholine to test vasoreactivity, which can only be administered during invasive coronary angiography. Thus, once non-invasive methodologies have ruled out obstructive disease, the European Society of Cardiology (ESC) guidelines specify diagnostic guidewire coronary function testing as a Class IIa recommendation to assess CFR, and further recommend intracoronary acetylcholine testing for microvascular spasm as a Class IIb recommendation.61 If VSA is considered, the ESC notes acetylcholine testing as a Class IIa recommendation.61
There is a paucity of clinical trials specifically relating to treatment and risk mitigation for patients with INOCA; however, medications should be tailored to specifically target the underlying cause. Women with INOCA identified to have plaque on imaging should be treated with anti-atherosclerotic therapies (i.e. statins and ACEI/ARB) to reduce the risk of MACE. Anti-anginal therapies are used to control symptoms. For individuals with microvascular dysfunction, β-blockers, calcium channel blockers or ACEI/ARB may help decrease workload and improve microvascular perfusion. Ranolazine may also significantly improve symptoms and quality of life in women with microvascular angina.62 Individuals with INOCA from VSA derive greater benefit from calcium channel blockers or long-acting nitrates.58
Treatment Modalities for Secondary Prevention of CVD
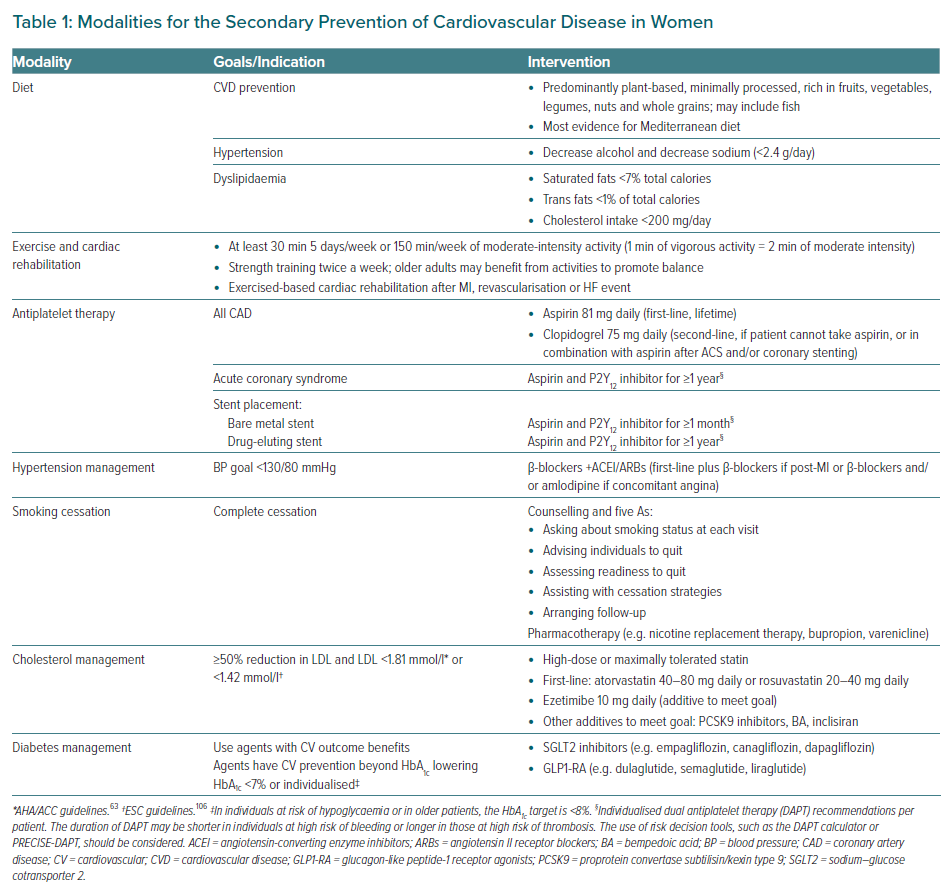
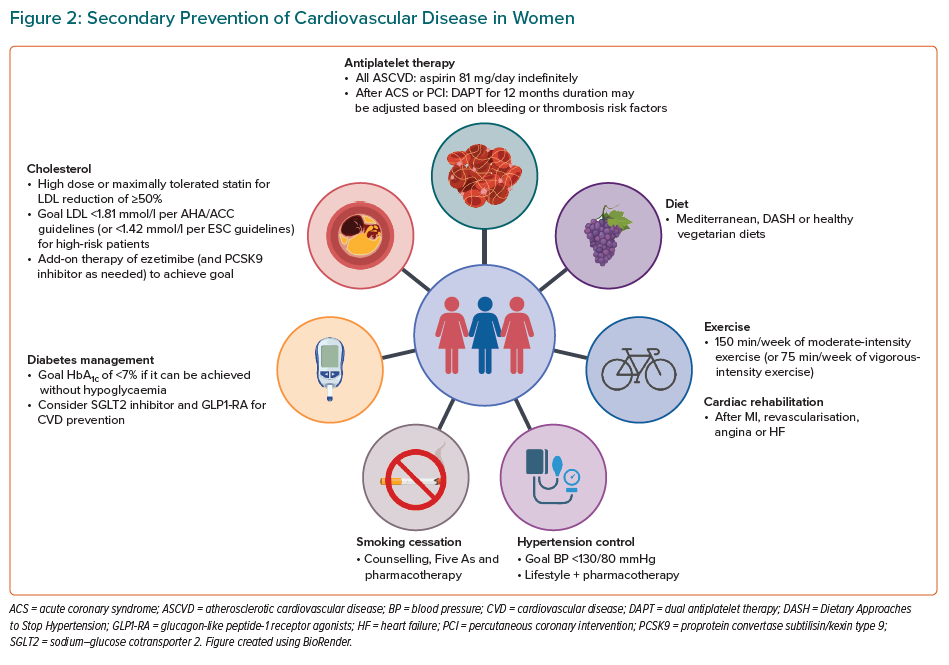
Treatment modalities for the secondary prevention of CVD in women are summarised in Table 1 and Figure 2, and discussed below.
Lifestyle Modifications
Diet
Lifestyle modifications are considered first-line treatment and are critical for mitigating the risk factors of CVD, including diabetes, lipids and blood pressure. The American Heart Association (AHA)/American College of Cardiology (ACC) guidelines recommend a diet that emphasises fruits, vegetables, whole grains, legumes, nuts and healthy protein sources, such as low-fat dairy, low-fat poultry (without the skin) and fish/seafood.63,64 It is recommended that the intake of red meat, sugar-sweetened beverages, sweets and highly refined grains is limited.63 Dietary patterns can be adapted to cultural preferences and to specific comorbid conditions (e.g. diabetes). It is recommended that saturated fats be replaced with healthy mono- and polyunsaturated fats.14 For those with high LDL cholesterol (LDL-C), fat intake goals are specified: saturated fats should account for <7% of total calories and trans fats for <1% of total calories, and cholesterol intake should be reduced to <200 mg/day.65 For individuals with hypertension, the recommendations also include minimising sodium and alcohol intake.65 Guidelines recommend sodium consumption of <2,400 mg/day, with a goal of 1,500 mg/day.65
The ESC guidelines provide similar recommendations to the ACC/AHA guidelines, with a particular endorsement of the Mediterranean diet.66 Among high-risk patients with stable CAD, adherence to the Mediterranean diet was associated with a reduced risk of MACE.67 Furthermore, in the Lyon Heart Study, post-MI patients who were randomised to the Mediterranean diet experienced a 72% reduction in recurrent non-fatal MI, as well as a 56% decline in mortality risk at 4 years of follow-up, compared with <30% in those randomised to a control low-fat diet.68
Across guidelines, the Dietary Approaches to Stop Hypertension (DASH) diet and Mediterranean diet are commonly recommended as heart healthy diets for hypertension and CVD, respectively.69 The DASH and Mediterranean diets share similar tenets of being primarily rich in fruits and vegetables and low in saturated fat or refined grains; a healthy plant-based diet would also align with these recommendations. The studied effects of these diets on secondary prevention are mixed and limited by difficulties with adherence; however, the use of a dietician has shown effectiveness in reducing risk factors.69–72 For individuals with a known CVD history or post-MI, having a high-quality diet is associated with a decreased risk of recurrent cardiovascular events and lower all-cause mortality respectively.73,74
Exercise and Cardiac Rehabilitation
Exercise improves metabolic control, cardiorespiratory fitness (leading to improvements in blood pressure and endurance) and is thought to improve myocardial function from weight loss.75 These effects are heightened when combined with dietary modifications and pharmacotherapy.76 The ESC and ACC/AHA guidelines both recommend regular physical activity, defined as at least 150 min/week of moderate aerobic exercise, divided into 30 min sessions 5 days a week, or 75 min/week of vigorous exercise for patients with known CVD and or peripheral artery disease.14,69,77 Moderate aerobic exercise has been defined as a brisk walk at 5–6 km/h and other equivalent exercises, such as swimming and biking, or house and garden work.78
CR programmes are AHA Class 1, Level A-recommended multidisciplinary and multifaceted programmes that often extend beyond exercise training to include diet, counselling, education and risk reduction.64 Compared with no exercise, exercise-based CR has been shown to reduce cardiovascular mortality after an acute event.77 Many CR programmes suffer from low enrolment and retention.79 Women are less likely to be referred and enrolled in CR programmes than men.39,80 Lack of familiarity with CR programmes, negative perceptions and a lack of transport, social support and systemic referral practices are causes of decreased enrolment numbers for women.39
CR is indicated after any ACS event or diagnosis of HF and is associated with overall increased survival, as well as improved functional status and psychosocial well-being and decreased hospitalisations.79 Women enrolled in CR programmes show an increase in exercise tolerance and decrease in body fat percentage similar to that of men, despite starting from a lower baseline, which further demonstrates the need for engagement.81
Antiplatelet Therapy
Following ACS or ischaemic stroke, all individuals are recommended to start aspirin 81–162 mg/day, which should be continued indefinitely for secondary prevention.64 The Antithrombotic Trialists conducted a meta-analysis that examined 16 secondary prevention trials with 17,000 individuals.82 Women were included in 14 of the 16 trials, making up an average of 10% of the sample population post-MI and 30% of the population post-transient ischaemic attack (TIA) or stroke.82,83 These studies did not show any evidence of sex interaction with aspirin efficacy for secondary prevention. Despite evidence for secondary prevention, women with stable ASCVD are 35% less likely than men to report being on aspirin therapy (OR 0.65; 95% CI [0.58–0.72]).37 For patients who are unable to tolerate aspirin, clopidogrel 75 mg/day is recommended as an alternative antiplatelet therapy.64
After stent placement, dual treatment with a P2Y12 inhibitor (such as clopidogrel, prasugrel or ticagrelor) and aspirin is recommended for ≥1 month in those in whom a bare metal stent was placed and ≥1 year after for those implanted with a drug-eluding stent.84 Other major trials compared prasugrel or ticagrelor to clopidogrel on a background of aspirin and showed similar results for men and women.83,85,86 Women made up approximately one-quarter of the population studied in these trials.
Although studies to date demonstrate no sex effect on the benefit of antiplatelet therapy in secondary prevention or on the adverse effects of antiplatelet therapy, women are overall under-represented in the majority of therapeutic trials. Meta-analyses suggest equal efficacy (i.e. ASCVD reduction) and safety (i.e. bleeding) between women and men and do not justify any differential dual antiplatelet therapy (DAPT) treatment by sex.87,88 Among individuals at high risk of bleeding after percutaneous coronary intervention (PCI), one emerging strategy is to stop aspirin after 3 months of DAPT and to continue with a P2Y12 inhibitor as monotherapy.89 In the TWILIGHT study, which tested this strategy, women did have higher bleeding rates after PCI than men, but this was largely due to differences in baseline characteristics.89 There was no difference between women and men with regard to the benefit of early aspirin withdrawal and ticagrelor monotherapy on ischaemic endpoints.89
Aspirin (81–325 mg) is also recommended both preoperatively and indefinitely after CABG to reduce the risk of cardiac events and graft occlusion.90 Clopidogrel 75 mg daily is the recommended alternative in the event that aspirin is not tolerated. One year of DAPT with aspirin and prasugrel or ticagrelor is a Class Ic recommendation for patients who initially present with ACS.91
The antiplatelet regimen to be used for women with a SCAD-type of MI, who are managed conservatively without PCI, is not well established given the absence of trials, and experts differ in their opinions. However, one large registry (DISCO) suggested worse outcomes at 1 year when DAPT was used instead of single antiplatelet therapy.92 This requires further evaluation and study, ideally through a randomised control trial.
Hypertension Control
Women tend to develop hypertension later in life than men, largely due from falling oestrogen levels after menopause. Recent US data suggest hypertension control has been getting worse in women since 2013.93 Although lifestyle changes, a low-sodium diet and weight control are regularly recommended for hypertension, individuals with a history of CVD and a blood pressure (BP) >130/80 mmHg should also be promptly started on antihypertensive medications for a target BP of <130/80.94
Initial therapy should be targeted to follow specific guidelines for CHD, previous MI, HF or stroke, as indicated. For example, β-blockers, ACEI or ARBs are first-line antihypertensive treatments in patients with a history of ASCVD; diuretics and mineralocorticoid receptor antagonists could also be considered as adjuvants for individuals with HF after ACEI or ARB.94 β-blockers have been shown to improve outcomes after CABG, particularly for individuals who initially presented with MI.90 There are no sex-related differences in drug classes for men versus women in BP lowering and cardiovascular risk protection.95 However, ACEI and ARBs are contraindicated for use during pregnancy due to teratogenicity and women should be transitioned to nifedipine, labetalol or methyldopa for BP management.94
One cross-sectional study from the National Health and Nutrition Examination Survey found that women were more likely to be on pharmacotherapy than men (61.4% versus 56.8%; p=0.001), but women were less likely to have BP controlled (44.8% versus 51.1%; p=0.018).96 The authors of that study hypothesised that this was a result of less intensive BP management for women, as evidenced by women being less likely to be on three or more antihypertensive agents.96
Smoking Cessation
Women have a 25% greater relative risk of a CVD event than men who smoke.97 Although predominantly associated with men, the number of young women who smoke, particularly in low- and middle-income strata, is on the rise. However, women are 50% more likely to be advised to quit than similarly aged men.98
Secondary prevention strategies for smoking cessation overlap with primary prevention recommendations; however, the necessity for quitting becomes more salient after an acute event and offers a fresh opportunity to discuss strategies with patients. Continuing to smoke after an MI confers an increased risk for a recurrent event.99 In contrast, cessation of smoking after an acute cardiovascular event has been shown to be associated with a 36% risk reduction in mortality from CHD and to decrease the risk of any recurrent vascular effect.100,101
Combined counselling and pharmacotherapy have proven effective for smoking cessation.102 Successful counselling strategies include group therapy, as well as individualised and telephone counselling. The ‘Five As’ strategy is a popular smoking cessation approach in the outpatient setting that involves:
- asking about smoking status at every visit;
- advising individuals to quit;
- assessing readiness to quit;
- assisting with cessation strategies; and
- arranging follow-up.
Nicotine-replacement therapy, bupropion and varenicline are among the pharmacotherapy options proven to aid smoking cessation.14
Cholesterol Management
As per the 2018 AHA/ACC/multisociety guideline for cholesterol management, all individuals <75 years with clinical ASCVD should be started on a high-intensity statin with the goal of reducing LDL-C by ≥50%.63 In individuals >75 years of age, at least moderate statin therapy should be initiated and up-titrated as tolerated. Women (compared with men) are less likely to be prescribed statins (67% versus 78%; p<0.001) and less likely to prescribed the appropriate guideline-recommended intensity of statin for their CVD (37% versus 45%; p<0.001).103
In a large meta-analysis of statin trials, among patients with vascular disease, statins reduced the risk of MACE similarly in women (RR 0.84; 95% CI [0.77–0.91]) and men (RR 0.79; 95% CI [0.76–0.82]; p for interaction by sex=0.43) for every 1 mmol/l reduction in LDL.26 After CABG, statins have also been recommended to slow the progression of disease in new grafts and, as per other secondary prevention recommendations, high-intensity statins should be used.90
Pregnant women should use statins during pregnancy and breastfeeding with caution due to a lack of cohesive data about the teratogenic effects of statins.104 However, it should be noted that recently the Food and Drug Administration (FDA) lifted its strongest warning label about statin use in pregnancy, which will allow clinicians some flexibility to consider this option for their highest-risk patients as part of shared decision-making.105
For secondary prevention, the 2018 AHA/ACC guidelines also recommend that if LDL-C remains above a threshold of 1.81 mmol/l after treatment with maximally tolerated statin, then ezetimibe should be added.63 If LDL still remains above 1.81 mmol/l despite maximally tolerated statin and ezetimibe, then a PCSK9 inhibitor, such as evolocumab or alirocumab, should be started.63 The ESC lipid targets are more aggressive, setting an LDL threshold goal of <1.42 mmol/l for high-risk patients.106 The FOURIER and ODYSSEY clinical trials evaluated PCSK9 inhibitor therapy among patients with either stable CHD or recent ACS, respectively.107,108 Both trials included approximately 25% women and demonstrated that adding PCSK9 inhibitors to a high-intensity statin decreased the risk of ischaemic CVD events, with similar benefits in women as in men.30
Novel approaches to dyslipidaemia continue to emerge. Bempedoic acid was recently approved by the FDA as an addition to maximally tolerated statin for patients with ASCVD if additional LDL lowering was needed.109,110 The cardiovascular outcomes trial for bempedoic acid is still underway. Women made up approximately 29% of the population studied in the published bempedoic acid Phase III trials but represent approximately half of population in the ongoing cardiovascular outcome trial for bempedoic acid (CLEAR Outcomes; NCT02993406).110 This further demonstrates that although women have been more robustly included in clinical trials over the past four decades, they continue to be under-represented.21–23,111 Inclisiran, a novel small interfering RNA inhibitor of PCSK9, has been demonstrated to reduce LDL by approximately 50% with twice-a-year injections.112 Approved in Europe, inclisiran is currently undergoing FDA regulatory approval.
Diabetes Management
Optimal glycaemic control is imperative for patients with known CVD, because type 2 diabetes (T2D) increases the risk of adverse events.113 The AHA guidelines recommend a target HbA1c of <7.0% for patients with a life expectancy of more than 10–20 years.114 The HbA1c goal could be liberalised to <8.0% or 8.5% in older patients if there is a risk of hypoglycaemia. More stringent glycaemic control has demonstrated a decreased risk of microvascular endpoints, with less certainty about macrovascular endpoints.114
For individuals with known atherosclerotic disease and T2D, the use of sodium glucose co-transporter 2 (SGLT2) inhibitors and glucagon-like peptide-1 receptor agonists (GLP1-RA) is recommended in conjunction with lifestyle modifications for CVD prevention.115 Note that the cardiovascular benefits conferred by SGLT2 inhibitors and GLP1-RA are independent of the HbA1c-lowering effects. SGLT2 inhibitors have been shown to reduce MACE, cardiovascular deaths and hospitalisations for HF, as well as to reduce the progression of chronic kidney disease.115–117 SGLT2 inhibitors confer similar CVD risk reduction in women as in men, with a similar safety profile.118
Meta-analyses of GLP1-RA in placebo-controlled trials demonstrated a reduction in MACE of approximately 12%, as well as a reduction in all-cause mortality and fewer admissions for HF.115,119 A meta-analysis of trials suggested that women and men experience similar benefit in ASCVD reduction with GLP1-RA.120 Real-world data suggest that women may benefit from GLP1-RA even more than men.121 GLP1-RA therapy also can confer meaningful weight loss reduction, which may be helpful for many patients with T2D. However, women only made up approximately 30–40% of the participants in the GLP1-RA trials.
Conclusion
Women are at high risk of secondary cardiovascular events and, compared with men, have poorer outcomes within the first 5 years. Mitigating risk and improving outcomes is dependent on:
- the proper identification of CVD in women through imaging and understanding atypical presentations;
- starting and titrating appropriate GDMT while also continuing to engage with lifestyle modifications; and
- increasing representation of women in cardiovascular clinical trials.
As our understanding of the CVD burden in women continues to grow, women are beginning to make up a more significant proportion of the studied population, which will allow us to further develop and tailor CVD guidelines and close the gap between diagnoses, treatment and mortality.