Venous thromboembolism (VTE) encompasses deep vein thrombosis (DVT) and pulmonary embolism (PE). Patients undergoing major surgery (especially major orthopaedic surgery) are prone to VTE, both symptomatic and asymptomatic, by activating all three components of Virchow’s triad (endothelial injury, stasis and hypercoagulability). The position of the limb during surgery, tourniquet use and prolonged post-operative immobilisation lead to venous stasis. Elevated pro-thrombotic factors, such as interleukin-6, C-reactive protein and tumour necrosis factor-α, induced by tissue injury, trigger tissue factor release and thrombin expression, platelet activation and initiate the coagulation cascade.1–3 Haemorrhage during surgery reduces antithrombin III levels and thus an imbalance between coagulation–fibrinolytic systems, exacerbating hypercoagulability.
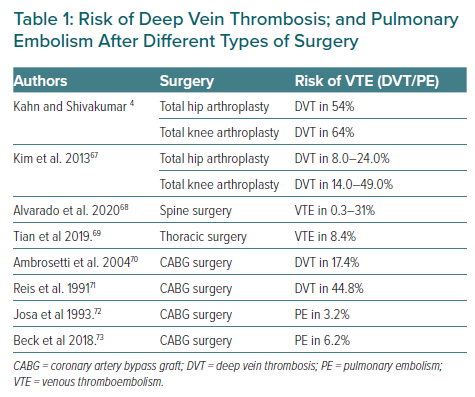
There are several risk prediction scores for VTE after major surgery.4 The incidence of DVT in clinical medicine and general surgery is 10–40%, compared to 40–60% in major orthopaedic surgery. In 2008, the American College of Chest Physicians (ACCP) classified the risks of VTE in hospitalised patients into three categories: low, moderate and high risk. Orthopaedic patients who have undergone hip or knee arthroplasty or sustained hip fracture, major trauma or spinal cord injury are included in the high-risk category.5 Table 1 summarises the risks of DVT and PE after different types of surgery.
Early mobilisation, along with mechanical and pharmacological prophylaxis, effectively reduce the risk of post-operative VTE. Traditional post-operative anticoagulation regimens include two steps: initial treatment with a rapidly acting parenteral anticoagulant, usually low-molecular-weight heparin (LMWH) 1 mg/kg/day, followed by an oral vitamin K antagonist (VKA), such as warfarin. The duration of warfarin treatment depends on the nature of the operation and the patient’s mobility status and prothrombotic risks. Hypercoagulability and impaired venous function can persist up to 6 weeks after surgery, indicating the necessity for extended post-operative thromboprophylaxis.6,7 However, LMWH and warfarin have some limitations, such as the need for daily injections, the risk of heparin-induced thrombocytopenia, regular dose monitoring, a narrow therapeutic window and various drug and food interactions. These limitations led to the development of direct oral anticoagulants (DOACs). With a rapid onset of action and predictable pharmacokinetic and pharmacodynamic profiles, DOACs can be prescribed in fixed doses without routine therapeutic monitoring, thus replacing parenteral anticoagulants and warfarin for VTE prophylaxis and treatment.8
Direct Oral Anticoagulants
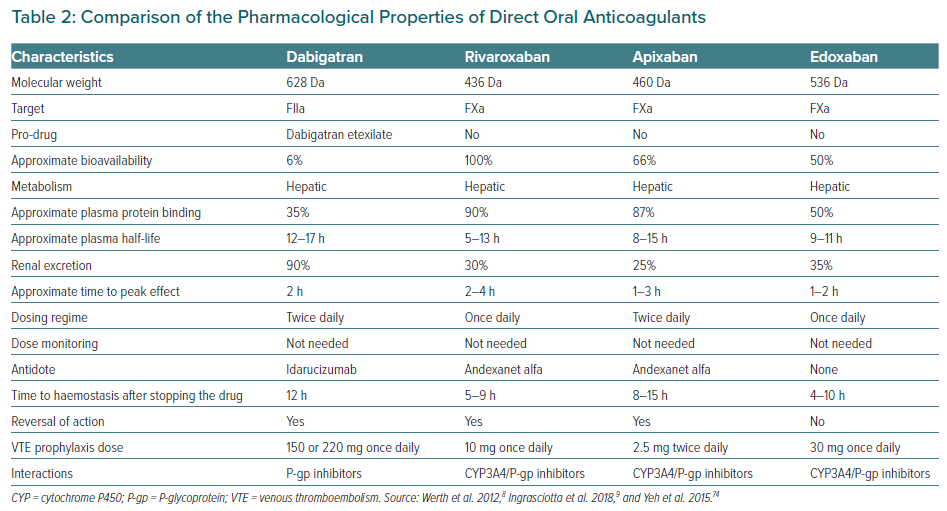
Unlike warfarin, which inhibits various steps in the coagulation cascade (vitamin K-dependent clotting factors II, VII, IX and X), DOACs target specific steps. They can be categorised into two broad groups: direct thrombin inhibitors (dabigatran) and selective factor Xa (FXa) inhibitors (rivaroxaban, apixaban and edoxaban). These commonly used DOACs are approved for post-operative VTE thromboprophylaxis in light of their favourable efficacy and safety profiles compared with LMWH and warfarin. In 2018, Ingrasciotta et al. studied the pharmacokinetics of DOACs and their clinical use.9 DOACs can be a better option than warfarin because of predictable pharmacokinetic properties, increased tolerability, fewer interactions and ease of use. However, because DOACs undergo hepatic metabolism and renal excretion, careful dose adjustment is required in people with hepatic or renal impairment. FXa inhibitors are contraindicated in those with creatinine clearance (CrCl) <15 ml/min, whereas dabigatran is contraindicated when CrCl is <30 ml/min. Table 2 compares the pharmacological properties of different DOACs.
General Recommendations for Venous Thromboembolism Prophylaxis
In 2015, a systematic review and meta-analysis by Ho et al. showed that VTE prophylaxis was associated with a reduced risk of PE (RR 0.45; 95% CI [0.28–0.72]; p=0.0008) or symptomatic VTE (RR 0.44; 95% CI [0.28–0.71]; p=0.0006). This review recommended initiating pharmacological VTE prophylaxis as soon as possible after cardiac surgery for patients who have no active bleeding.10 Sarker et al. reported that combined treatment with rivaroxaban and heparin is of great clinical value in post- coronary artery bypass grafting (CABG) deep vein thrombosis (DVT) patients.11 A 2-year (2015–2016) retrospective cohort analysis comparing LWMH and DOACs for thromboprophylaxis in operative spinal trauma patients showed that DOAC thromboprophylaxis was associated with less chance of DVT than LMWH (1.8 versus 7.4%, respectively) and PE (0.3 versus 2.1%, respectively).12 Analysis from the National Joint Registry for England Wales, Northern Ireland and Isle of Man compared DOACs to aspirin in 218,650 total hip arthroplasty (THA) and total knee arthroplasty (TKA) patients, finding that DOACs were associated with a lower risk of VTE.13
In 2011, the American Academy of Orthopaedic Surgeons recommended the use of pharmacological agents and/or mechanical compressive devices for the prevention of VTE in patients undergoing elective hip or knee arthroplasty (grade of recommendation: moderate).14 The ACCP’s 2012 guidelines suggest the use of mechanical devices (intermittent pneumatic compression devices) plus pharmacological prophylaxis during hospitalisation in patients at high risk for VTE after major orthopaedic surgery.15 The guidelines recommend apixaban, dabigatran and rivaroxaban for a minimum of 10–14 days, and up to 35 days for VTE prophylaxis in patients undergoing THA or TKA (grade of recommendation: grade 1B, strong, moderate quality).15 The National Institute for Health and Care Excellence (NICE) 2019 guideline recommends apixaban, rivaroxaban, dabigatran for VTE prevention after THA or TKA.16 The American Society of Hematology (ASH) 2019 guideline suggests apixaban, rivaroxaban, dabigatran over LMWH for VTE prevention after THA or TKA (conditional recommendation based on moderate certainty in the evidence of effects).17 The Scottish Intercollegiate Guidelines Network (SIGN) 2014 recommends rivaroxaban or dabigatran, combined with mechanical prophylaxis unless contraindicated, in patients undergoing THA or TKA (grade A recommendation).18 Moreover, the 2019 European Society of Cardiology guidelines on PE recommend DOACs (apixaban, dabigatran, edoxaban or rivaroxaban) in preference to VKA (recommendation Class I, level of evidence A) for acute-phase treatment of intermediate or low-risk PE.19
Direct Thrombin Inhibitors
Dabigatran
Dabigatran etexilate is the pro-drug of dabigatran. Dabigatran selectively blocks the activity of thrombin and is mainly (90%) eliminated by kidneys, so dose adjustment should be considered those with renal insufficiency. The usual dosage of dabigatran is 220 mg once daily or 150 mg once daily if CrCl is 30–50 ml/min. It is contraindicated if CrCl <30 ml/min. Four Phase III trials (RE-NOVATE, RE-NOVATE II, RE-MODEL, RE-MOBILIZE) have compared the efficacy and safety of dabigatran with enoxaparin for VTE prophylaxis after THA or TKA.20–23 In all four trials, the primary efficacy outcome was total VTE events (symptomatic or venographic DVT and/or symptomatic pulmonary embolism) and all-cause mortality during treatment. The primary safety outcome was the occurrence of bleeding events (major, clinically relevant non-major bleeding and minor bleeding events).
In the randomised, double-blind, non-inferiority RE-NOVATE trial, a total of 3,494 patients undergoing THA were randomised to 220 or 150 mg dabigatran once daily or enoxaparin 40 mg once daily for 28–35 days. The primary efficacy outcome (reducing the risk of a VTE) occurred in 6.0% of those receiving dabigatran 220 mg, 8.6% of those receiving dabigatran 150 mg and 6.7% of those receiving enoxaparin. Major bleeding events were detected in 2.0%, 1.3% and 1.6%, respectively.20 In RE-NOVATE II (also a randomised, double-blind, non-inferiority trial), comprising 2,055 patients who underwent THA, extended (28–35 days) prophylaxis with dabigatran 220 mg once daily was as effective as enoxaparin 40 mg once daily in reducing risk of total VTE and all-cause mortality (dabigatran 7.7% versus enoxaparin 8.8%; risk difference 1.1; 95% CI [−3.8, 1.6]) with p<0.0001 for non-inferiority, and similar safety profiles.21
The randomised, double-blind, non-inferiority RE-MODEL trial examined dabigatran 150 or 220 mg once daily versus enoxaparin 40 mg once daily for 6–10 days in 2,076 patients who underwent TKA. Dabigatran 220 mg or 150 mg had similar efficacy and safety profiles compared to enoxaparin for VTE prophylaxis after TKA. Total VTE and all-cause mortality occurred in 36.4% of those receiving dabigatran 220 mg, 40.5% of those receiving dabigatran 150 mg, and 37.7% of those receiving enoxaparin. Major bleeding occurred in 1.5%, 1.3% and 1.3%, respectively.22 The fourth Phase III trial, the RE-MOBILIZE trial, compared dabigatran 220 mg or 150 mg once daily versus enoxaparin 30 mg twice daily in 1,896 patients undergoing TKA for 12–15 days. Although dabigatran is effective when compared to once-daily enoxaparin, this trial demonstrated that dabigatran showed inferior efficacy to twice-daily enoxaparin.23
The pooled analysis of three of the trials (RE-NOVATE, RE-MODEL, RE-MOBILIZE) did not show any difference in efficacy and safety profiles between the dabigatran and enoxaparin groups.24 The BISTRO II trials showed that dabigatran was effective and safe across a range of doses (dabigatran 50 mg twice daily, 150 mg twice daily, 300 mg once daily and 225 mg twice daily) compared to enoxaparin 40 mg once daily.25 In 2016, Rosencher et al. conducted an international, open-label, prospective, observational, single-arm study of dabigatran 220 mg once daily in over 5,000 patients undergoing THA or TKA. The data supported the safety and efficacy findings of previous dabigatran Phase III trials.26 Moreover, in 2015, Wurning et al. proved that switching from LMWH to dabigatran was safe and effective for VTE prophylaxis after THA or TKA.27
Based on these trials, dabigatran is recommended by ACCP, NICE, ASH and SIGN for DVT prevention after THA (28–35 days) and TKA (10 days).15–18 However, dabigatran has not been studied in hip fracture surgery.
Factor Xa Inhibitors
Rivaroxaban
Rivaroxaban is a Food and Drug Administration (FDA) approved oral direct factor Xa inhibitor for prevention of thromboembolism after THA and TKA that requires no routine laboratory monitoring. The safety and efficacy of rivaroxaban was studied in the RECORD study program, which is composed of four separate randomised, double-blind, Phase III clinical trials (RECORD 1, 2, 3 and 4).28–31 The primary efficacy endpoint in all RECORD trials was total VTE, symptomatic or asymptomatic DVT, non-fatal PE and all-cause mortality.
In the RECORD 1 and RECORD 2 trials, which included a total of 7,050 patients undergoing THA, rivaroxaban 10 mg once daily was superior to enoxaparin 40 mg once daily for VTE prophylaxis with similar safety profiles.28,29 In the RECORD 3 trial, involving 2,531 patients who underwent TKA, a 10–14 day course of rivaroxaban 10 mg once daily significantly reduced the incidence of VTE compared to enoxaparin 40 mg once daily (rivaroxaban 9.6% versus enoxaparin 18.9%; p<0.001) without increasing bleeding events.30 The fourth RECORD trial, RECORD 4, compared a 10–14 day course of rivaroxaban 10 mg once daily with enoxaparin 30 mg twice daily in 3,148 patients undergoing TKA. Rivaroxaban was significantly superior to twice-daily enoxaparin (rivaroxaban 6.9% versus enoxaparin 10.1%; p=0.0118) for the prevention of VTE after TKA.31
A pooled analysis of the four RECORD trials proved that, compared with enoxaparin (either enoxaparin 40 mg once daily or enoxaparin 30 mg twice daily), rivaroxaban 10 mg once daily reduces the incidence of VTE and all-cause mortality after elective THA or TKA (rivaroxaban 0.5% versus enoxaparin 1.0%; p=0.001), with a small increase in bleeding.32 In 2014, Levitan et al. conducted a post hoc analysis to assess the benefit–risk profile for rivaroxaban versus enoxaparin in the RECORD studies, which showed rivaroxaban resulted in greater benefits than harms compared with enoxaparin.33 The ODIXa-HIP and ODIXa-KNEE studies showed that rivaroxaban 2.5–10 mg twice daily has favourable efficacy and safety profiles compared to enoxaparin for prevention of VTE after THA or TKA.34,35
In 2014, Turpie et al. conducted the XAMOS, Phase IV, non-interventional, open-label cohort study to assess the safety and effectiveness of rivaroxaban compared with other pharmacological VTE prophylaxis (standard of care; SOC). The crude incidence of symptomatic VTE was 0.89% in the rivaroxaban group versus 1.35% in the SOC group (OR 0.65; 95% CI [0.49–0.87]). This study confirmed that rivaroxaban has a favourable benefit–risk profile compared to SOC after major orthopaedic surgery.36 Moreover, the Ortho-TEP registry showed that rivaroxaban was associated with fewer VTE and bleeding events than fondaparinux in patients undergoing major orthopaedic surgery.37 In 2020, Smith et al. evaluated that prolonged (35-day) prophylaxis with rivaroxaban is cost effective for VTE prophylaxis after TKA.38 Again, Sarker et al. reported that combined treatment with rivaroxaban and heparin is of great clinical value in post-CABG DVT patients.11
Based on these trials, rivaroxaban has been recommended by ACCP, NICE, ASH and SIGN for DVT prevention after THA and TKA.15–18 The recommended dosing is rivaroxaban 10 mg once daily with the first dose administered 6–10 hours post-surgery for 28–35 days (after THA) or 10–14 days (after TKA). However, rivaroxaban has not been studied in hip fracture surgery.
Apixaban
Apixaban is an oral direct FXa inhibitor, approved by the FDA for thromboembolism prophylaxis after THA and TKA. Apixaban does not require routine laboratory monitoring for its anticoagulant effect, but it is contraindicated in patients with severe renal impairment (CrCl <15 ml/min). Apixaban was evaluated in the ADVANCE study programs (ADVANCE 1, 2 and 3), which compared apixaban with enoxaparin. All trials were randomised, double-blind, double-dummy, non-inferiority, Phase III trials. In these trials, enoxaparin was started 12 hours pre-operatively and apixaban was started 12–24 hours after wound closure. The primary efficacy outcome was the incidence of symptomatic or asymptomatic DVT, non-fatal PE, or all-cause mortality during treatment. The primary safety outcome was the incidence of bleeding events (major or clinically relevant non-major bleeding).
In the ADVANCE 1 trial, a 10–14-day course of apixaban 2.5 mg twice daily was compared with enoxaparin 30 mg twice daily for VTE prophylaxis in 3,195 patients undergoing TKA. The primary efficacy endpoint was reached in 9.0% of the apixaban group versus 8.8% of the enoxaparin group (RR 1.02; 95% CI [0.78–1.32]; p=0.06 for non-inferiority). Bleeding risk was significantly lowered in apixaban-treated patients (2.9% versus 4.3% for apixaban versus enoxaparin respectively; p=0.03). Therefore, apixaban did not meet the prespecified statistical criteria for non-inferiority, despite the low bleeding risk.39 The ADVANCE-2 and ADVANCE-3 trials compared apixaban 2.5 mg twice daily with enoxaparin 40 mg once daily in patients undergoing TKA and THA, respectively. The prophylaxis was continued for 10–14 days after TKA and 35 days after THA. The ADVANCE-2 trial showed incidence of VTE was significantly reduced in the apixaban (15%) versus the enoxaparin (24%) group (RR 0.62; 95% CI [0.51–0.74]; p<0.0001). Bleeding events occurred in 4% of the apixaban group and 5% of the enoxaparin group (p=0.09).40 In the ADVANCE-3 trial, the primary efficacy endpoint was reached in 1.4 versus 3.9% of the apixaban- and enoxaparin-treated patients, respectively (RR 0.36; 95% CI [0.22–0.54]; p<0.001 for both non-inferiority and superiority). Major and clinically relevant non-major bleeding was not different between the two groups (apixaban 4.8% versus enoxaparin 5%).41
In 2012, Raskob et al. conducted a pooled analysis of the ADVANCE 2 and ADVANCE 3 trials that included 8,464 patients. VTE events were statistically lower in the apixaban (0.7%) group versus the enoxaparin (1.5%) group (risk difference, apixaban minus enoxaparin, −0.8%; 95% CI [−1.2, −0.3]; one-sided p<0.0001 for non-inferiority; two-sided p=0.001 for superiority) without increasing bleeding risk (risk difference −0.6; 95% CI [−1.5, 0.3]). It was concluded that apixaban 2.5 mg twice daily is more effective than enoxaparin 40 mg once daily without increasing bleeding events.42 The APROPOS trial was a randomised, eight-arm, parallel group, multi-centre, Phase II trial, that compared different doses of apixaban (5, 10 or 20 mg once daily or 2.5, 5 or 10 mg twice daily) with enoxaparin or warfarin titrated to an international normalized ratio 1.8–3.0 in patients undergoing TKA. Apixaban 2.5 mg twice daily or 5 mg once daily has a favourable benefit–risk profile compared with SOC (enoxaparin or warfarin).43 A meta-analysis and trial-sequential analysis of four trials (APROPOS, ADVANCE 1, 2 and 3) concluded that apixaban 2.5 mg twice daily seems equally effective and safe to LMWH twice daily, and superior to with LMWH once daily.44 In 2019, a study by Torrejon Torres et al. revealed that apixaban or intermittent pneumatic compression, or a combination of the two, is the most cost-effective for VTE prophylaxis after lower limb arthroplasty.45
Based on these trials, apixaban is recommended by the ACCP, NICE and ASH for DVT prevention after THA and TKA.15–17 Currently, apixaban is approved in the EU for the prevention of VTE in patients undergoing major orthopaedic surgery at a dose of 2.5 mg twice daily commencing 12–24 hours after surgery for 10–14 days (knee replacement surgery) and 32–38 days (hip replacement surgery). However, apixaban has not been studied in hip fracture surgery. Therefore, apixaban is not currently recommended for hip fracture surgery.
Edoxaban
Edoxaban is an oral, direct, FXa inhibitor. It does not require routine monitoring of therapeutic effect but it is contraindicated in severe renal impairment (CrCl 15–30 ml/min). Three Phase II dose-ranging studies showed that compared to placebo, enoxaparin or dalteparin, edoxaban has a statistically significant (p<0.001) dose-dependent reduction in VTE events in patients undergoing major orthopaedic surgery with a similar bleeding risk.46–48
The STARS program (STARS-E3, STARS-J4 and STARS-J5) compared the efficacy and safety of edoxaban 30 mg once daily with enoxaparin 20 mg twice daily in patients undergoing major orthopaedic surgery. The prophylaxis was given for 11–14 days following surgery. The primary efficacy endpoint was the incidence of VTE. Safety endpoints were the incidence of bleeding events, major, or clinically relevant non-major bleeding. In the STARS-E3 trial, a randomised, double-blind, non-inferiority, Phase III trial, 716 patients undergoing TKA were randomised to either edoxaban or enoxaparin. VTE occurred in 7.4% of those receiving edoxaban versus 13.9% for enoxaparin; relative risk reduction 46.8%; p<0.001 for non-inferiority and p=0.010 for superiority.49 The STARS-J4 trial was a multi-centre, randomised, open-label, active-comparator, Phase III trial that studied 92 patients undergoing hip fracture surgery. The incidence of thrombotic events was 6.5% in the edoxaban group and 3.7% in the enoxaparin group. Major and clinically non-relevant minor bleeding occurred in 3.4% of the edoxaban group and 6.9% of the enoxaparin group.50 Another randomised, double-blind, non-inferiority, Phase III trial, STARS-J5, studied 610 patients undergoing THA. The efficacy outcome occurred in 2.4% of the edoxaban group versus 6.9% of the enoxaparin group (relative risk reduction 65.7%; p<0.001 for non-inferiority). Bleeding occurred in 2.6% of edoxaban-treated patients versus 3.7% of enoxaparin-treated patients; p=0.475.51 In a pooled analysis of the STARS-E3 and STARS-J5 trials, the incidence of VTE was 5.1% and 10.7% for edoxaban and enoxaparin, respectively, p<0.001. There was also no significant difference in bleeding rates (4.6% for edoxaban and 3.7% for enoxaparin, p=0.427).52 Based on these results, edoxaban has recently been approved for VTE prophylaxis after major orthopaedic surgery in Japan at a dose of 30 mg once daily.53
Comparison Between Direct Oral Anticoagulants
Zhang et al. conducted a retrospective study to compare the efficacy and safety of apixaban and rivaroxaban after lumbar spine surgery. A total of 480 patients were randomised to apixaban 2.5 mg twice daily or rivaroxaban 10 mg once daily for 14 days. All patients were provided with graduated compression stockings for 6 weeks, and calf-length intermittent pneumatic compression devices while in-hospital with mobilisation encouraged. VTE events, bleeding and D-dimer changes were assessed. There was no significant intergroup difference in the incidences of thrombotic events between apixaban (5%) and rivaroxaban (3.75%), p>0.05. Total bleeding and minor bleeding were significantly lower in the apixaban group (p<0.05). Moreover, postoperative D-dimer level changes were lower in the apixaban group than in the rivaroxaban group. Therefore, apixaban and rivaroxaban were equally effective for post-operative VTE prophylaxis.54
A systematic review and meta-analysis has been conducted comparing dabigatran, rivaroxaban and apixaban versus enoxaparin for DVT prophylaxis after THA or TKA. The meta-analysis included 38,747 patients from 16 Phase II and Phase III trials.55 Compared with enoxaparin, VTE risk was lower with rivaroxaban (RR 0.48; 95% CI [0.31–0.75]), and similar with dabigatran (RR 0.71; 95% CI [0.23–2.12]) and apixaban (RR 0.82; 95% CI [0.41–1.64]). However, the risk of bleeding was higher with rivaroxaban (RR 1.25; 95% CI [1.05–1.49]), similar with dabigatran (RR 1.12; 95% CI [0.94–1.35]), and lower with apixaban (RR 0.82; 95% CI [0.69–0.98]).55
In 2017, a network meta-analysis was conducted to compare the efficacy and safety of anticoagulants for VTE prevention after hip and knee arthroplasty. The outcomes revealed that rivaroxaban and apixaban were superior to enoxaparin for reducing VTE. Rivaroxaban was associated with similar bleeding risks compared with enoxaparin 30 mg twice daily and higher bleeding risks compared with enoxaparin 40 mg once daily. However, apixaban was associated with a decreased major or clinically relevant non-major bleeding compared with either dose of enoxaprin.56
Three meta-analyses have demonstrated that DOACs (dabigatran, apixaban and rivaroxaban) reduce the risk of VTE compared to placebo. Based on these studies, apixaban may have the most favourable efficacy and safety profiles for post-operative VTE prophylaxis. However, there are no direct comparative trials between different types of DOACs, so a definite opinion on whether apixaban is the best DOAC cannot be made regarding these data.57–59
Bleeding Risks of Direct Oral Anticoagulants
Bleeding (major and minor) is the most common complication of DOACs. A population-based cohort study showed that the risk of gastrointestinal bleeding with DOACs (dabigatran and rivaroxaban) was similar to warfarin.60 Chai-Adisaksopha et al. performed a systematic review and meta-analysis of twelve randomised controlled trials. The bleeding risk of DOACs was assessed in 102,607 patients with VTE or AF. Compared with VKAs, DOACs significantly reduced the risk of overall major bleeding (RR 0.72; p<0.01), fatal bleeding (RR 0.53; p<0.01), intra-cranial bleeding (RR 0.43; p<0.01), clinically relevant non-major bleeding (RR 0.78; p<0.01) and total bleeding (RR 0.76; p<0.01).61
Management of Direct Oral Anticoagulants in Perioperative Settings
The management of patients taking DOACs in the perioperative setting is important. The pharmacokinetic properties of DOACs, renal function, bleeding risks, nature of the surgical procedure and thromboembolic risk of patients should all be considered.62 Although periprocedural bridging anticoagulation with LMWH or unfractionated heparin has been used in some high-thromboembolic risk-patients, a systematic review and meta-analysis proved that there was no difference in thromboembolic risk between bridged and non-bridged patients (RR 1.26; 95% CI [0.61–2.58]; p=0.53). However, bridging anticoagulation increased risk of overall bleeding (RR 2.83; 95% CI [2.00–4.01]; p<0.0001) and major bleeding (RR 3.00; 95% CI [1.78–5.06], p<0.0001).63
Dabigatran undergoes 90% renal elimination. In high-bleeding-risk procedures, it is recommended to discontinue dabigatran 48–72 hours prior to surgery in patients with normal renal function or mild impairment (CrCl >50 ml/min), 72–96 hours with moderate renal impairment (CrCl 30–49 ml/min) and 96–144 hours with severe renal impairment (CrCl <29 ml/min). In low-bleeding-risk procedures, dabigatran does not need to be interrupted if renal function is normal. Dabigatran should be resumed between 48–72 hours after high-bleeding-risk procedures and 24 hours after low-bleeding-risk procedures. Rivaroxaban should be discontinued 48 hours prior to high-bleeding-risk procedures. In low-bleeding-risk procedures, rivaroxaban should be withheld 24 hours prior to surgery with normal renal function (CrCl >90 ml/min), 48 hours with mild renal impairment (CrCl 60–90 ml/min), 72 hours with moderate renal impairment (CrCl 30–59 ml/min), and 96 hours with severe renal impairment (CrCl 15–29 ml/min). Rivaroxaban can be restarted as soon as after haemostasis is achieved in low-bleeding-risk procedures and after 48–72 hours in high-bleeding-risk procedures. For apixaban, it is recommended that it is withheld for 24–48 hours with mild renal impairment (CrCl <60 ml/min), 72 hours with moderate renal impairment (30–59 ml/min) and 96 hours with severe renal impairment (CrCl <30 ml/min) in high-bleeding-risk procedures. In low-bleeding-risk procedures, apixaban may be continued without interruption. Following surgery, apixaban may be resumed after 24–48 hours depending on bleeding risks. Edoxaban is suggested to be discontinued 24 hours prior to low-bleeding-risk procedures and 72 hours prior to high-bleeding-risk procedures. During prolonged gaps without anticoagulation, bridging anticoagulation with heparin may be considered in high thromboembolic risk patients, although a meta-analysis does not support this regime.62
If emergency surgery cannot be delayed for at least 12 hours from the last DOAC intake, specific reversal agents should be considered. A randomised, double-blind, placebo-controlled study showed prothrombin complex concentrate immediately and completely reverses the anticoagulant effect of rivaroxaban in healthy subjects but has no influence on the anticoagulant action of dabigatran.64 Idarucizumab was approved in 2015 as the specific reversal agent for dabigatran and andexanet alfa (a recombinant modified FXa protein) was approved in 2018 for the reversal of the anticoagulation action of rivaroxaban and apixaban in cases of life-threatening or uncontrolled bleeding, or where rapid reversal of anticoagulation is required.65,66
Conclusion
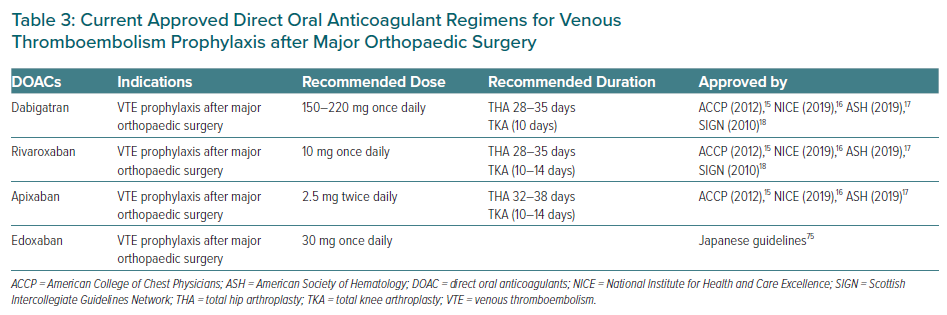
In summary, based on these above clinical data, DOACs have similar or superior efficacy and safety profiles compared to routine SOC (LMWH and warfarin) for VTE prophylaxis after major orthopaedic surgery. Table 3 summarises the current approved DOACs guidelines for VTE prophylaxis after major orthopaedic surgery. However, the data regarding the role of DOACs after non-orthopaedic surgery are limited. Therefore, regarding post-operative VTE prophylaxis, the risks of thromboembolism and bleeding should be assessed and managed on an individual basis to obtain optimal outcomes.