Despite advances in cardiovascular research, coronary artery disease (CAD) remains the leading cause of death and disability in developed nations.1 Atherosclerosis has traditionally been associated with risk factors, such as smoking, dyslipidaemia, arterial hypertension and diabetes.2–4 However, in recent years, inflammation of the arterial wall has emerged as a key mechanism in the development of this condition.5
Given the involvement of inflammatory mechanisms in atherogenesis, attempts have been made to identify circulating inflammatory biomarkers that can predict future cardiovascular events. These biomarkers include C-reactive protein (CRP), serum amyloid A protein, neopterin, lipoprotein-associated phospholipase A2, pro-inflammatory cytokines, matrix metalloproteinases, heat shock proteins and adhesion molecules.6 These biomarkers and the molecular pathways that generate them are the targets in a new field of anti-inflammatory therapeutics for primary and secondary cardiovascular prevention.
This review will explore the clinical use of CRP, the most frequently studied biomarker in this context; the concept of residual risk in primary and secondary cardiovascular prevention; and the current recommendations in the main international clinical practice guidelines regarding the role of this inflammatory biomarker in cardiovascular risk stratification.
Inflammatory Markers in Atherosclerosis
Coronary atherosclerosis has been traditionally associated with well-known cardiovascular risk factors, which form the basis of the current approach toward controlling the CAD pandemic.2–4 However, it has become apparent that inflammation has a key role in atherogenesis and its complications.5 Atherogenesis represents an inflammatory process with cytokine production and increased blood levels of acute phase reactants similar to that observed in other inflammatory diseases, such as rheumatoid arthritis. Inflammatory cell infiltration is observed in atherosclerotic plaques at all stages of the disease, from the first fatty streak to advanced atheromatous lesions and thrombotic complications.
There is a huge amount of evidence implicating inflammation in atherosclerosis and acute coronary syndromes (ACS) and a variety of circulating markers of inflammation have been examined for their ability to predict either the presence of vascular disease or the risk of vascular events in a broad range of clinical settings.7 These markers included CRP, serum amyloid A, fibrinogen, neopterin, lipoprotein-associated phospholipase A2, soluble CD40 ligand, heat shock proteins, matrix metalloproteinases, myeloperoxidase, pro-inflammatory cytokines and many circulating adhesion molecules.6 The identification of vulnerable plaques in vulnerable patients using imaging techniques or inflammatory biomarkers is one of the most promising areas of research in modern cardiology and could revolutionise cardiovascular practice.
C-reactive Protein
Of the various inflammatory biomarkers described to date, CRP has been the most frequently studied.8 CRP is a member of the family of pentraxins, soluble pentameric proteins that recognise microbial structures and play an essential role in innate immunity. These pentraxins act as pattern recognition receptors capable of recognising pathogen-associated molecular patterns, repetitive structures evolutionarily preserved in microorganisms. CRP binds somatic C-polysaccharide of Streptococcus pneumoniae, a polysaccharide that is rich in lysophosphatidylcholine (LPC), the natural ligand of CRP.9,10 CRP also binds to phosphocholine expressed on the cell membrane of apoptotic cells.
CRP has a pentameric structure composed of five identical 23-kDa polypeptide subunits non-covalently associated in a cyclic symmetry.9,10 It is produced primarily in the liver as an acute-phase reactant in response to inflammatory or ischaemic tissue damage following the local or systemic production of pro-inflammatory cytokines, such as interleukin (IL)-1β, IL-6 or tumour necrosis factor-α by the nucleotide-binding and oligomerisation domain-, leucine-rich repeat- and pyrin domain-containing protein 3 (NLRP3) inflammasome.
The features that have made CRP an attractive biomarker of chronic inflammation are its long half-life, its stable circulating levels, its minimal circadian variation, and the availability of affordable and validated high-sensitivity methods for its determination.8,11 CRP is an independent prognostic marker in patients with atherosclerotic disease and in apparently healthy subjects.12,13
Primary Prevention
Inflammatory markers have a prognostic value for the development of cardiovascular events independent of conventional risk factors and may be useful for identifying people who are at high risk of future cardiovascular events and may benefit from specific treatment to reduce this risk.
A meta-analysis that included 160,309 patients without a previous history of cardiovascular disease confirmed that each standard deviation increase in high sensitivity (hs) CRP was associated with increased adjusted relative risk of CAD, ischaemic stroke and cardiovascular death of 37% (95% CI [1.27–1.48]), 27% (95% CI [1.15–1.40]) and 55% (95% CI [1.37–1.76]), respectively.14 This same study showed that the magnitude of such risk was comparable with that associated with traditional cardiovascular risk factors associated with the development of CAD, including total cholesterol (16%), non-HDL cholesterol (28%) and arterial systolic blood pressure (35%).
The REGARDS study confirmed the prognostic value of CRP in primary prevention for patients at high risk of cardiovascular disease, defined as Framingham coronary risk score ≥10% or atherosclerotic cardiovascular disease (ASCVD) risk ≥7.5%).15 Of the 6,136 high-risk patients in this study, those with high LDL cholesterol (LDL-C; ≥1.8 mmol/l) and low hs-CRP (<2 mg/l) had a lower risk of incident stroke (HR 0.69; 95% CI [0.47–0.997]), incident CAD (HR 0.71; 95% CI [0.53–0.95]), and cardiovascular death (HR 0.70; 95% CI [0.50–0.99]), whereas low LDL-C (<1.8 mmol/l) was not associated with protective effects. These results support the role of inflammation in atherogenesis and plaque instability. In the PRINCE study, assignment to 40 mg/day of pravastatin reduced CRP concentrations by 16.9% (p<0.001) at 24 weeks, regardless of lipid profile, providing evidence of the anti-inflammatory properties of statins in addition to their lipid-lowering effects.16 However, it is still unknown whether this reduction in CRP levels is associated with a decrease in cardiovascular risk and whether CRP could be used to guide statin therapy.
In the AFCAPS/TexCAPS study, baseline CRP concentrations were determined in 5,742 apparently healthy subjects at low or moderate risk for the development of coronary events.17 After a mean follow-up of 5.2 years, 20–40 mg/day lovastatin reduced the occurrence of first acute major coronary event (fatal or non-fatal MI, unstable angina or sudden death from cardiac causes) in subjects with elevated levels of LDL-C (>3.86 mmol/l; RR 0.53; 95% CI [0.37–0.77]), but it also reduced these events in patients with elevated CRP concentrations (>0.16 mg/dl) and normal LDL-C values (RR 0.58; 95% CI [0.34–0.98]).
The JUPITER study analysed the effectiveness of rosuvastatin in reducing major cardiovascular events in 17,802 apparently healthy subjects with normal cholesterol levels (<3.4 mmol/l), but with high CRP concentrations (≥2 mg/l).18 The study was prematurely stopped after a median follow-up of 1.9 years. Rosuvastatin significantly reduced the incident of major cardiovascular events (combined endpoint of MI, stroke or death from cardiovascular causes) in these apparently healthy subjects without hyperlipaemia but elevated hs-CRP levels (HR 0.53; 95% CI [0.40–0.69]).
These results confirm the usefulness of CRP and lipid profile for the assessment of cardiovascular risk in primary prevention. They demonstrate that achieving both lipid and inflammatory targets significantly improves prognosis compared with the achievement of just one of these therapeutic aims in isolation.8
Secondary Prevention
Stable Coronary Artery Disease
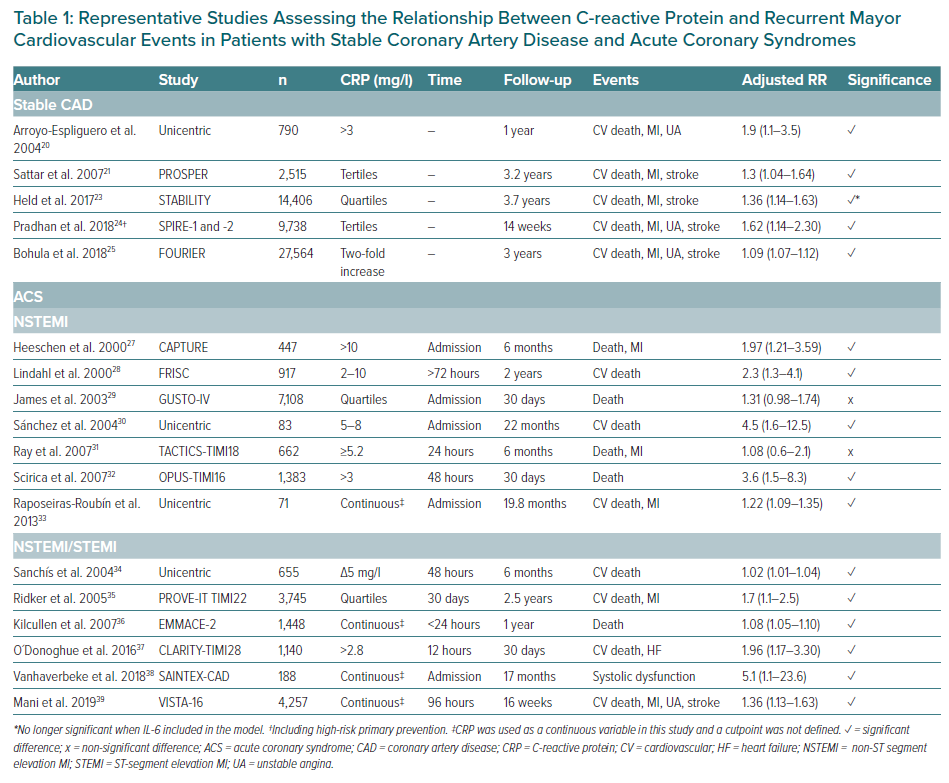
CRP has been associated with the development of recurrent cardiovascular events in patients with stable CAD (Table 1), both in prospective cohort studies and in analyses of clinical trials.19–25 These studies demonstrated the association of CRP with the risk of MI, the need for revascularisation, stroke and heart failure, and cardiovascular, cancer-related and total mortality.
In a single-centre study of 700 consecutive patients with chronic stable angina (CSA) who underwent scheduled coronary revascularisation, serum hs-CRP levels were significantly associated with the development of the combined endpoint of cardiac death, non-fatal acute MI or hospital admission with unstable angina at 1-year follow-up (OR 1.9; 95% CI [1.1–3.5]), regardless of age, sex, previous MI, type 2 diabetes and the extent or severity of CAD.20
In the 13,740 patients with CSA and LDL-C ≥1.8 mmol/l on a statin assigned to placebo in the FOURIER study, those with higher baseline hs-CRP categories had significantly higher 3-year Kaplan-Meier rates of the combined endpoint of cardiovascular death, MI, stroke, hospitalisation for unstable angina or coronary revascularisation: 12.0%, 13.7% and 18.1% (ptrend<0.0001) for categories <1, 1–3, and >3 mg/dl, respectively.25
Many of the studies that confirm the predictive capacity of hs-CRP for the development of recurrent events have been carried out in populations treated with pro-protein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, suggesting that hs-CRP can identify residual inflammatory risk even in patients with very low LDL-C levels.24,25 Studies with intravascular ultrasound show that the reduction in hs-CRP is accompanied by a significant decrease in the progression of atherosclerotic plaque, whether or not serum lipid concentrations are modified. In fact, reductions in LDL-C and hs-CRP concentrations are associated with a greater deceleration of atherosclerotic progression than reductions in only one of these markers.26
Acute Coronary Syndromes with or without ST-segment Elevation
Numerous clinical studies have demonstrated the ability of CRP to predict recurrent coronary events in patients with ACS (Table 1).27–39 Liuzzo et al. showed that elevation of CRP at hospital admission predicted a poor outcome in patients with unstable angina. Patients who had levels of CRP ≥0.3 mg/dl had more ischaemic episodes (MI, cardiac death and urgent coronary revascularisation) during hospital admission.40 CRP was also predictive of cardiac risk of mortality and MI (18.9% versus 9.5%; p=0.003) at 6-month follow-up in the 447 patients with unstable angina enrolled in the placebo arm of the CAPTURE trial.27
Hs-CRP concentrations increase rapidly after ACS, reaching a peak at 48–96 hours, with a progressive return to baseline levels over the following weeks.41 The measurement of hs-CRP in the acute phase of an ACS may reflect the combination of the low-grade systemic inflammation that triggers the atherosclerotic plaque rupture and the inflammatory response secondary to myocardial ischaemia and necrosis.19 Patients with non-ST segment elevation MI tend to have more stable hs-CRP concentrations in the acute phase of the coronary condition, given the lower degree of inflammation after myocardial necrosis in these patients.42
The origin of the elevated hs-CRP levels in patients with ACS is unknown. In fact, the correlation between markers of myocardial necrosis (troponins) and CRP is weak.43 CRP appears to be a marker of hyperresponsiveness of the inflammatory system to even minimal stimuli. The increases in CRP and IL-6 concentration observed after vascular damage in acute MI or coronary angioplasty correlated with baseline CRP and IL-6 levels, which suggest that only those patients with high baseline CRP or IL-6 concentrations showed increased CRP values after vascular damage caused by angioplasty.44 This individual difference in response to inflammatory stimuli may have a genetic basis; certain haplotypes in the IL-1/IL-1R gene complex correlate with the sustained inflammatory response and the incidence of CAD.45
However, despite the weak correlation between markers of infarct size and hs-CRP, its predictive value for the development of recurrence is complementary and additive.43 Based on the OPUS TIMI-16 study, the risk of death at 30 days in 450 patients with ACS rose from 1% when CRP, troponin I, and B-type natriuretic peptide were negative to approximately 6% when all markers were positive.46
Hs-CRP concentrations can remain elevated for weeks after an acute coronary event, especially in patients with ST-segment elevation.45,46 Studies such as PROVE IT-TIMI 22 and Aggrastat-to-Zocor (A-to-Z) have shown that hs-CRP measured 30 days after the ACS event is an independent predictor of mortality and recurrent MI.47, 48 Moreover, an analysis of the VISTA-16 trial found that a higher baseline hs-CRP level (HR 1.36; 95% CI [1.13–1.63]) and a higher longitudinal hs-CRP level (HR 1.15; 95% CI [1.09–1.21]) were independently associated with major cardiovascular adverse events (composite of cardiovascular death, MI, non-fatal stroke, or unstable angina with documented ischaemia requiring hospitalisation).39 As suggested by this study, serial measurements of hs-CRP 16 weeks after an ACS event may help to identify those patients at higher risk of mortality and morbidity.
Severity, Extent and Activity of Atherosclerosis
No clear association has been found between CRP concentrations and the severity and extent of coronary atherosclerosis. Zebrack et al. reported that CRP concentration did not correlate with the number of severe (≥70%) or moderate (10–70%) coronary lesions in 2,554 patients with symptomatic ischaemic heart disease who had undergone coronary angiography. In fact, the risk of death or MI at 5 years in patients with high CRP concentrations (>2 mg/dl) and normal coronary arteries (11%) was higher than the risk in patients with low CRP concentrations (<1 mg/dl) and severe coronary disease (8%).49
Other studies have confirmed the independent and complementary predictive value of hs-CRP and the extent of coronary atherosclerosis for the development of cardiovascular events.20,22 Patients with hs-CRP and CAD extension score above the median had a five times higher risk of cardiac death and non-fatal MI at 1-year follow-up than patients with lower than median values (OR 5.0; 95% CI [2.3–10.6]). Therefore, the independent relationship between CRP and cardiovascular adverse events in patients with ischaemic heart disease suggests that clinical stability does not always reflect atherosclerotic plaque stability.
Katritsis et al. found that CRP concentrations were correlated with the complex angiographic morphology of the atherosclerotic plaque, specifically with the presence of intracoronary thrombus and eccentric/irregular discrete morphology.50 Other studies also confirm the correlation of hs-CRP, neutrophil count and neopterin with the number of angiographically complex lesions in patients with ACS.51 These inflammatory markers have also been associated with rapid progression of atherosclerosis. Hs-CRP and intercellular adhesion molecule-1 showed independent associations with rapidly progressive coronary disease, increasing the risk of its development fivefold (15% versus 75%; ptrend<0.001) if both markers are elevated (hs-CRP >3 mg/l and intercellular adhesion molecule-1 >271.4 ng/ml).52 Similar results were obtained in the GENERATION study, where CRP showed an independent association with the progression of CAD in previously untreated lesions at 1-year follow-up.53 These results confirm that endothelial dysfunction of inflammatory origin plays an important pathogenic role in the progression of coronary disease.
CRP is a marker of inflamed, vulnerable and unstable atheroma plaque, but not of its severity or extent. The measurement of inflammatory markers can help clinicians to identify patients who are likely to suffer an inflammatory process capable of triggering an acute coronary event or the development of rapidly progressive atherosclerosis.
The Biological Functions of C-reactive Protein
Cardiovascular Risk Factor or Marker?
Historically, CRP appeared to be directly involved in the different phases of the atherogenic process, from endothelial activation to the erosion or disruption of the atheroma plaque.54,55 However, the current belief is that all these biological effects of CRP were due to contamination by endotoxins or to the use of recombinant CRP of bacterial origin.56 Studies with highly purified human CRP have not demonstrated any biological effects. In fact, the inhibition of CRP synthesis with an antisense oligonucleotide was accompanied by an endotoxin-mediated decrease in CRP production, but there were no alterations in other components of the inflammatory response.57 These studies confirm that pentameric CRP (pCRP) is more of a marker of inflammatory processes than a causative factor for these processes.
However, these studies do not rule out a potential causal pro-inflammatory effect of non-circulating monomeric CRP (mCRP) in atherosclerosis. In fact, CRP monomers may be responsible for enhancing the inflammatory response in tissues after pCRP dissociation on the surface of activated platelets or damaged or apoptotic cells. Modification of the phospholipid composition of the cell membrane mediated by PLA2, released in inflamed or ischaemic tissues, may lead to the exposure of LPC, a natural ligand of pCRP, allowing the release of mCRP. These CRP monomers, predominantly located in tissue, may enhance inflammatory mechanisms, favouring the transendothelial migration of leukocytes, monocyte activation through Fcγ-RI/III receptors and the activation of complement inhibitors factor H and C4b-BP.58
The neutral results with pCRP infusion coincide with the data obtained from Mendelian randomisation studies which confirm that CRP is a predictor of cardiovascular events but is unlikely to play a direct aetiopathogenic role in the inflammatory processes associated with atherosclerosis, in contrast to the powerful evidence for IL-6 or IL-1β, which also seem to have a causative role in the pro-inflammatory process.59,60 Hs-CRP may be the surrogate biomarker for the activation of this pro-inflammatory pathway mediated by IL-1β and IL-6. As a result, translational pharmacological studies are currently focusing on the inhibition of the pathway mediated by the activation of the inflammasome NRLP3/IL-1β and IL-6. CRP appears to be a clinically ideal biomarker for monitoring the inhibition of this pro-inflammatory pathway and the potential reduction of recurrent cardiovascular events associated with its pharmacological inhibition.
Residual Inflammatory Risk
Despite adequate control of risk factors and even achieving the recommended therapeutic goals in secondary prevention, the 10-year risk of recurrence of major cardiovascular events in patients with atherosclerosis is 10–30%.61 There are pathophysiological mechanisms that explain a significant part of this residual risk of recurrence that persists even in patients with adequately controlled LDL-C.19
In the FOURIER study, the median rate of events during the 26 months of follow-up in patients receiving evolocumab, who presented a mean LDL-C of 0.78 mmol/l, was 9.8%.62 In the GLAGOV study, up to one-third of the patients receiving combined treatment of evolocumab and statins presented increased atheroma volume despite reaching a mean LDL-C of 0.95 mmol/l.63 These observations suggest that even with the significant reduction in lipid levels achieved by PCSK9 inhibitors there is a residual risk of atherosclerosis progression and recurrence of major cardiovascular events.
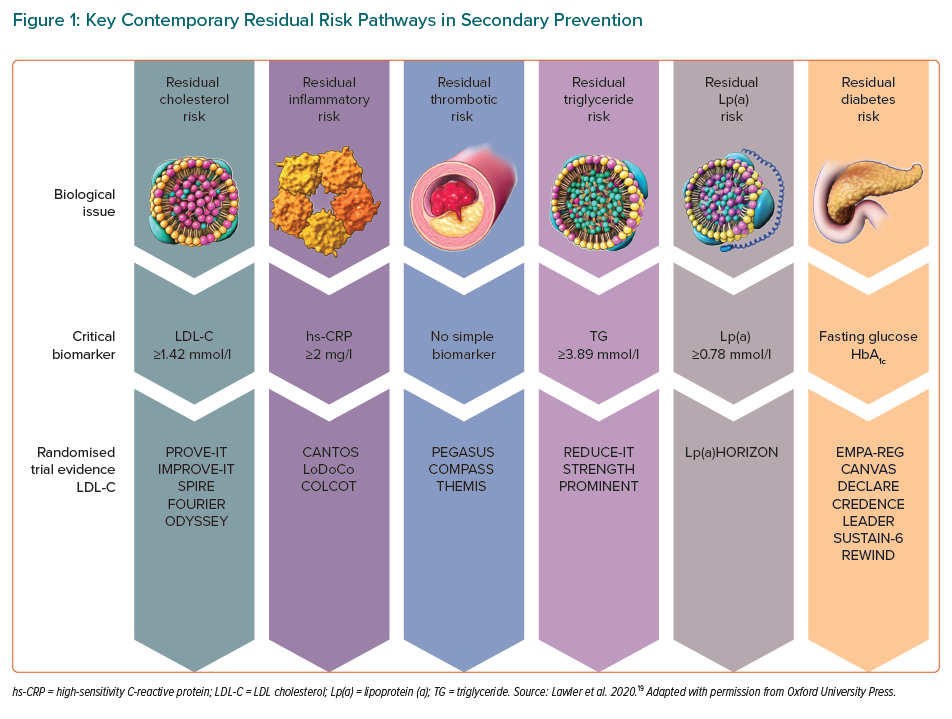
Efforts to reduce this residual risk further have focused on enhancing the reduction of LDL-C, lipoprotein-a, triglicerides, prothrombosis, hyperglycaemia, and persistent subclinical arterial inflammation (Figure 1).19 This persistent vascular inflammation is one of the most important pathophysiological mechanisms in the development of recurrent cardiovascular events.
High Potency Statins ± Ezetimibe
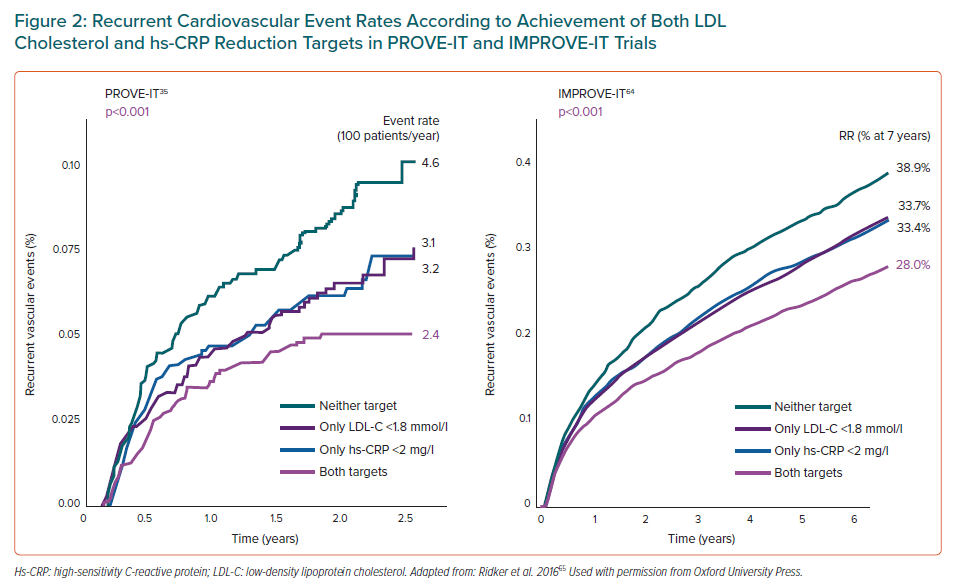
The prevalence of this residual inflammatory risk (RIR) in secondary prevention has been analysed in several clinical studies. In the PROVE IT-TIMI 22 study, patients with RIR, defined by an LDL-C <1.8 mmol/l and hs-CRP >2 mg/l (44% of the total), presented an age-adjusted annual event rate of 3.1% compared to 2.4% in patients with hs-CRP <2 mg/l.35 In the IMPROVE-IT study, patients with LDL-C <1.8 mmol/l and hs-CRP> 2 mg/l (33% of all patients) presented a crude event rate of 33.7% at 7-year follow-up (defined as cardiovascular death, heart attack or non-fatal stroke) compared to 28% in patients with a hs-CRP <2 mg/l. In this same study, the combination of simvastatin and ezetimibe achieved the dual objective of controlling LDL-C <1.8 mmol/l and hs-CRP <2 mg/l in 50% of patients, while the use of simvastatin alone achieved this in only 29% of the patients, since ezetimibe produced a significant reduction of 0.3 mg/l in hs-CRP compared to the baseline value.64 These studies confirm that achieving the dual goal of lipid control (LDL-C <1.8 mmol/l) and inflammatory control (hs-CRP <2 mg/l) is accompanied by a significant reduction in the residual risk of cardiovascular events (Figure 2).65
PCSK9 Inhibitors
Large-scale studies with PCSK9 inhibitors have demonstrated this RIR in the development of cardiovascular events. In the SPIRE1 and 2 studies involving bococizumab, hs-CRP also identified patients with a higher risk of events despite adequate lipid control. Patients with hs-CRP >3 mg/l (34.9% of the total) had a cardiovascular event rate of 3.59 per 100 person years, compared to 1.96 in those with hs-CRP <1 mg/l, corresponding to adjusted HR of 1.62 (95% CI [1.14–2.30]) after adjustment for cardiovascular risk factors and on-treatment levels of LDL-C.24
The FOURIER study reported a 59% decrease in LDL-C concentrations using evolocumab, without any change in hs-CRP concentrations.62 Even so, patients with hs-CRP >3 mg/l presented a greater reduction in events during treatment – absolute risk reduction 2.6% (HR 0.80; 95% CI [0.71–0.90]), compared to 1.8% (HR 0.93; 95% CI [0.83–1.05]) in patients with hs-CRP 1–3 mg/l and 1.6% (HR 0.82; 95% CI [0.70–0.95]) in those with <1 mg/l.25 Even in patients achieving very low LDL-C levels (<0.52 mmol/l) 1 month after randomisation, those with a hs-CRP >3 mg/l had a 3-year primary event rate of 13.1% (95% CI [10.8–15.3]) compared to those with hs-CRP <1 mg/l (9.0% [95% CI [7.4–10.6]).25 Again, these large-scale studies with PCSK9 inhibitors identify hs-CRP as a predictor of cardiovascular events even in patients with very low LDL-C concentrations.
In all these studies, RIR was usually more frequent in women or in patients with metabolic syndrome/diabetes, high blood pressure, heart failure, peripheral arterial disease, chronic kidney disease ≥G3a (eGFR <60 ml/min/1.73 m2), a TIMI risk score in secondary prevention indicating high risk (≥4), or who were smokers.25
Percutaneous Coronary Intervention
The prognostic value of hs-CRP for predicting the recurrence of cardiovascular events has also been demonstrated after percutaneous coronary intervention (PCI).66 The single-centre study by Guedeney et al. included 3,013 patients undergoing PCI with a baseline LDL-C concentration of ≥1.8 mmol/l. RIR was defined as the persistence of hs-CRP concentrations >2 mg/l in the clinical stability phase – at least 4 weeks after an ACS event, the index PCI, or any intercurrent infectious/inflammatory process. Persistent high RIR (baseline and follow-up hs-CRP >2 mg/l) was recorded in 34.1% of patients and was associated with a higher risk of serious cardiovascular events (cardiovascular death, heart attack and non-fatal stroke) at 1-year follow-up (adjusted HR 2.10; 95% CI [1.45–3.02]) with an incidence rate of 152.4 (95% CI [126.0–184.4]) per 1,000 person years.
In summary, RIR is one of the factors involved in the development of recurrent cardiovascular events. Large-scale studies on secondary prevention confirm that up to one-third of patients with coronary atherosclerosis present RIR, defined as the presence of hs-CRP concentrations >2 mg/l. This RIR is associated with a significantly increased risk of major cardiovascular events, regardless of baseline LDL-C concentrations. Even in patients with extremely low baseline LDL-C concentrations (LDL-C <0.52 mmol/l) undergoing treatment with PCSK9 inhibitors, hs-CRP can identify those with RIR and therefore improve prognostic stratification.
The achievement of the dual goal of lipid and inflammatory control is accompanied by a further reduction in major cardiovascular events. Lipid-lowering drugs bring down LDL-C levels and significantly reduce the recurrence of major cardiovascular events. Unlike PCSK9 inhibitors, statins and ezetimibe also reduce hs-CRP concentrations but despite their lack of effect on hs-CRP values, PCSK9 inhibitors reduce the recurrence of cardiovascular events in patients with RIR, as borne out by the results obtained with evolocumab in the FOURIER study. Until specific anti-inflammatory or immunomodulatory drugs become part of the therapeutic arsenal against atherosclerosis, lipid-lowering drugs can help to reduce lipid and inflammatory residual risks in primary and secondary prevention (Table 2).
Anti-Inflammatory Treatments (Immunomodulators) in Atherosclerosis
The lack of a specific immunomodulatory treatment that interferes with the pro-inflammatory mechanisms involved in atherosclerosis has restricted the clinical use of inflammatory biomarkers – especially hs-CRP – in risk stratification. Numerous immunomodulatory treatments that interfere with the different pro-inflammatory pathways involved in atherosclerosis, such as the humanised anti-LDL-ox antibody, succinobucol, darapladib, varespladib, losmapimod, methotrexate, anakinra and inclacumab, have been analysed in clinical trials, with negative results.67–75
However, the beneficial clinical effect in reducing recurrent major cardiovascular events with canakinumab and colchicine, which are directed against the pro-inflammatory signalling pathway of the NLRP3/IL-1b inflammasome, have renewed the interest in inflammation as an aetiopathogenic mechanism of atherosclerosis.
Canakinumab
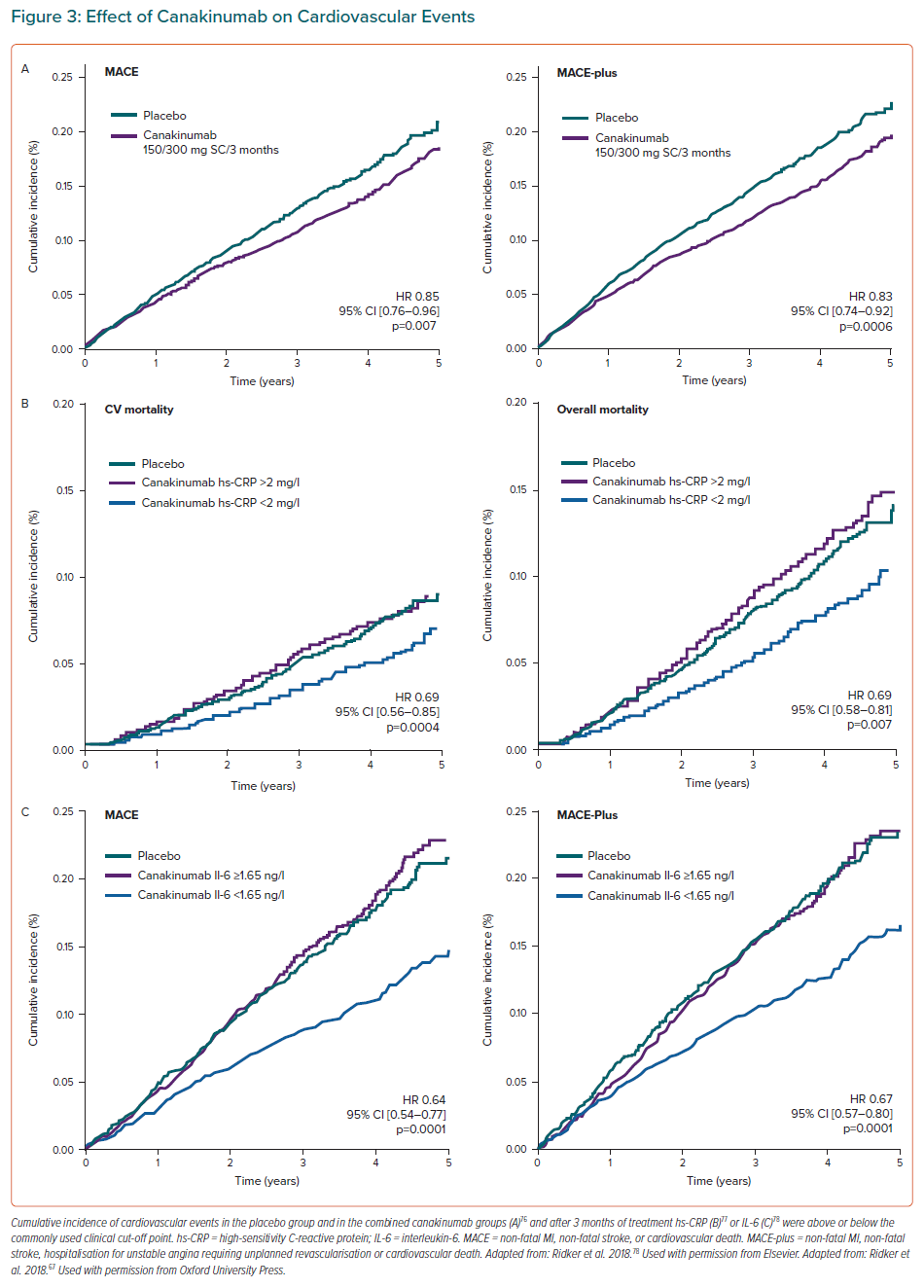
Canakinumab is a humanised monoclonal antibody directed against IL-1β. It is used to treat rheumatoid arthritis, gout and cryopryrin-associated periodic syndromes. In the CANTOS study, 10,061 patients with stable CAD, previous MI and hs-CRP concentrations of ≥2 mg/l were randomised to receive canakinumab at doses of 50 mg, 150 mg and 300 mg in quarterly subcutaneous injections versus placebo over a mean follow-up period of 3.7 years.76 The primary endpoint of the study was the combination of cardiovascular death, MI or non-fatal stroke. Canakinumab at a dose of 150 mg was associated with a significant reduction of 15% in the risk of the combined primary endpoint (HR 0.85; 95% CI [0.74–0.98]).76 After 48 months of treatment, reductions in hs-CRP of 35–40% were observed without any change in LDL-C concentrations. The patients who benefited from canakinumab treatment were those with reduced hs-CRP concentrations, known as cytokine responders. Patients with hs-CRP <2 mg/l 3 months after treatment had a significant 25% reduction in the risk of developing the combined endpoint (multivariable adjusted HR (HRadj) 0.75; 95 CI [0.66–0.85]).These patients also presented a 31% reduction in the risk of cardiovascular mortality (HRadj 0.69; 95% CI [0.56–0.85]) and all-cause mortality (HRadj 0.69; 95 CI [0.58–0.81]).77 The decrease in innate immunity recorded with the use of canakinumab was associated with a higher risk of fatal infections and sepsis (0.31 versus 0.18 events in the placebo group per 100 person years), mainly due to Gram-positive microorganisms (Table 2).76
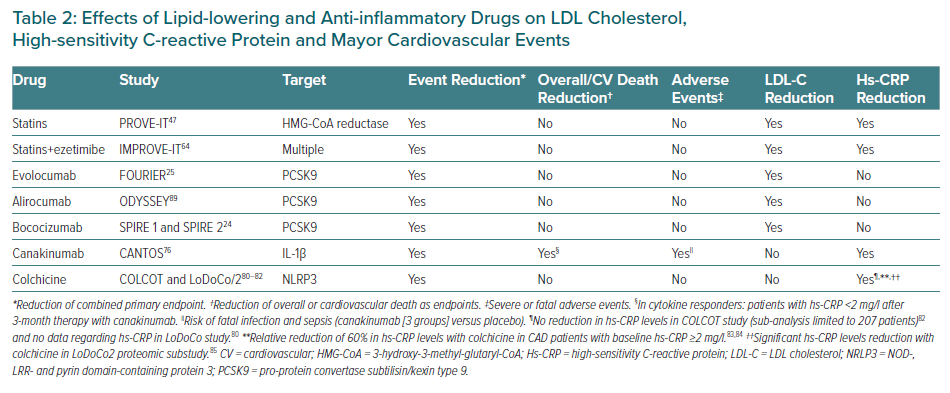
In summary, the results of the CANTOS study demonstrate that inhibition of IL-1β is accompanied by a significant reduction in recurrent cardiovascular events in patients with coronary artery disease and a high RIR (hs-CRP >2 mg/l). The study confirms that the reduction of events runs in parallel to the reduction of inflammatory biomarkers, such as hs-CRP and IL-6 (Figure 3).78 Identifying RIR can help tailor available treatments to the specific risk profile of each patient and personalise cardiovascular therapy based on the predominant residual risk.
Colchicine
Colchicine is an alkaloid that derives from the autumn crocus. Its mechanism of action is not entirely clear, but it is known to bind to tubulins, interfering with mitotic spindles and causing microtubule depolymerisation. Thus, colchicine interferes with processes that depend on the adequate polymerisation of microtubules, such as chemotaxis, phagocytosis and the activation of nuclear factor (NF)-kB or the NLRP3 inflammasome.79 The LoDoCo study included 532 patients with stable coronary artery disease (>6 months) receiving 0.5 mg daily of colchicine versus placebo. After a mean follow-up of 3 years, there was a significant 67% reduction (HR 0.33; 95% CI [0.18–0.59]) in the combined criteria of ACS, out-of-hospital cardiac arrest and non-cardioembolic stroke, mainly caused by the reduction of ACS unrelated to stent disease.80 The recently published follow-up study LoDoCo2 randomised 5,522 patients with stable coronary disease >6 months to colchicine 0.5 mg daily versus placebo. After a mean follow-up period of 28.6 months, there was a 31% reduction in the combined endpoint of cardiovascular death, MI and coronary revascularisation secondary to myocardial ischaemia (HR 0.69; 95 CI [0.57–0.83]).81
The COLCOT study analysed 4,745 patients 30 days after an MI randomised to 0.5 mg daily of colchicine versus placebo with a mean follow-up of 22.6 months.82 The primary endpoint was the combination of cardiovascular death, recovered cardiac arrest, MI or stroke, and unstable angina requiring urgent revascularisation. Colchicine achieved a 23% reduction in the risk of major events (HR 0.77; 95% CI [0.61–0.96]), with an increased risk of pneumonia (0.9% versus 0.4% of those in the placebo group; p=0.03). The patients had a baseline hs-CRP of 4.28 mg/l. No significant differences were observed in the reduction of hs-CRP at 6 months after the index event between colchicine versus placebo, but data for hs-CRP were only available for 207 patients. However, colchicine was shown to reduce hs-CRP concentrations by up to 60% in patients with atherosclerosis and baseline hs-CRP levels > 2 mg/l, regardless of the use of aspirin and atorvastatin.83,84 A proteomic substudy of LoDoCo2 demonstrated reductions in mean CRP concentrations from 1.52 mg/l to 1.0 mg/l (p<0.001), and in NLRP3 inflammasome-dependent proteins and neutrophil activity, confirming the important and varied anti-inflammatory effect of colchicine in atherosclerosis (Table 2).85
Current Recommendations
The clinical practice guidelines of the main scientific societies state that determining hs-CRP levels is a useful measure for primary cardiovascular prevention. However, the European Society of Cardiology (ESC) guidelines say that the determination of circulating biomarkers did not add relevant prognostic information to the prediction of cardiovascular risk obtained with the SCORE index, and so their measurement was not recommended in primary prevention – class of recommendation (COR): III; level of evidence (LOE): B.3 The 2011 ESC guidelines on the management of dyslipidaemia considered that the determination of hs-CRP could add prognostic information in patients with intermediate risk according to the SCORE index (≥1% and <5%), but the update in 2019 did not establish specific recommendations for its clinical use in primary prevention.86,87
In the 2019 American Heart Association (AHA) guidelines, hs-CRP is considered an enhancer of cardiovascular risk, so its determination is recommended for moderate risk patients (≥7.5% and <20% in the ASCVD risk index) for the initiation or intensification of lipid-lowering treatment with statins (COR: IIa; LOE: B–R).4 The guidelines establish an indication IIa (LOE: B-R) for the determination of hs-CRP. In these cases, the presence of an hs-CRP level of 2 mg/l would warrant the start of lipid-lowering treatment with moderate intensity statins (COR: I; LOE: A) in order to bring LDL-C down to 30–49% (COR: I; LOE: A). Despite the recommendations regarding hs-CRP in primary prevention, there are no specific guidelines for its determination in secondary prevention. The ESC consensus statement on inflammation in atherosclerosis concludes that CRP determination is not advised as it adds little value to the existing methods of cardiovascular risk assessment.88
Persistent elevation of hs-CRP despite adequate control of LDL-C has been shown to indicate the presence of RIR, but the absence of clinical pharmacology studies demonstrating a reduction in events with exclusively anti-inflammatory drugs has restricted the generalisation of the determination of hs-CRP in patients with CAD. However, the CANTOS study showed that the inhibition of IL-1β reduces recurrent cardiovascular events in patients with cardiovascular disease and persistent inflammation (hs-CRP ≥2 mg/l).76 The identification of new therapeutic targets will allow the development of drugs that are likely to help reduce the high residual risk of recurrence of cardiovascular events in patients with CAD.
Conclusion
Atherosclerosis is a chronic inflammatory disease. Among the biological markers of this inflammatory vascular process, the most frequently studied to date – and the most clinically useful – is CRP. CRP is a prognostic marker for mortality and recurrence of cardiovascular events for primary and secondary prevention; it identifies a group of patients with residual inflammatory cardiovascular risk despite adequate LDL-C control. Achieving control of both LDL-C and CRP is associated with a greater reduction in the risk of adverse cardiovascular events than adequate control of LDL-C alone. However, despite the evidence of the usefulness of CRP for improving risk stratification in primary and secondary prevention, it remains underused in clinical practice.
Advances in research on inflammatory mechanisms in atherosclerosis should provide new insights into the pathophysiology of the disease and help to define the aetiopathogenic factors involved in its development and the occurrence of plaque instability. The clinical studies in progress should allow a definitive assessment of the clinical use of CRP in primary and secondary prevention. The identification of new therapeutic targets in this field of clinical research will also promote the development of new drugs able to reduce the high residual risk of recurrence of major cardiovascular events in patients with coronary artery disease.