Coronary artery disease (CAD) is the leading cause of death in most countries. Compelling evidence from epidemiological, genetic and clinical studies as well as experiments in animal models has unquestionably established that elevated concentrations of cholesterol (mainly transported by LDL particles) promote atherosclerotic lesions.1 Although statin-based lipid-lowering therapies have been shown to reduce major CV events, even after a strong reduction in LDL cholesterol levels there is still a significant residual risk that cannot be ignored. Despite continuous advances in the treatment of acute and chronic coronary syndromes with catheter- and pharmacotherapy-based interventions, additional therapies are needed to reduce the rate of recurrent CV events, which remains too high.2,3
Inflammation and Atherosclerosis: New Insights into an Old Story
Classically, atherosclerosis was considered to be a degenerative disease caused by the continuous accumulation of cholesterol in the arterial intima. Furthermore, the idea that atherosclerosis is a predominantly lipid-driven disease has dominated the field of CV diseases for many years. However, over the last few decades, the concept of atherogenesis has changed as a result of new evidence that atherosclerosis is linked to a chronic low-grade inflammation of the vessel wall. In fact, the notion that atherosclerosis carries features of an inflammatory disease has been suspected since the 19th century, based on pathological observations made by Rudolf Virchow, Karl Rokitansky and others.4 The concept that inflammation may play an important role in atherosclerosis is one that has grown in parallel with the pathology itself for more than a century. Nevertheless, it is only in recent years that chronic inflammation has become recognised as a pivotal factor in the development of CAD. Dr Russell Ross, in a classic review on mechanisms of atherosclerosis in 1999, stated: “Atherosclerosis, an inflammatory disease”.5
It is recognised that in atherosclerosis, inflammation starts and evolves in response to cholesterol accumulation in the arterial intima of the large and medium arteries. However, new insights into innate immunity have shifted the understanding of the events that initiate and drive the inflammation. This has changed several concepts regarding the pathogenesis of the inflammatory disorders and made it clear that innate and adaptive immune responses play a pivotal role throughout the initiation, progression, and clinical consequences of atherosclerotic diseases. It is now known that one of the initial stages involves endothelial cell activation and the recruitment of inflammatory cells to the vessel wall, leading to a wide array of monocyte-derived macrophages, among other cells and pro-inflammatory cytokines.6–8
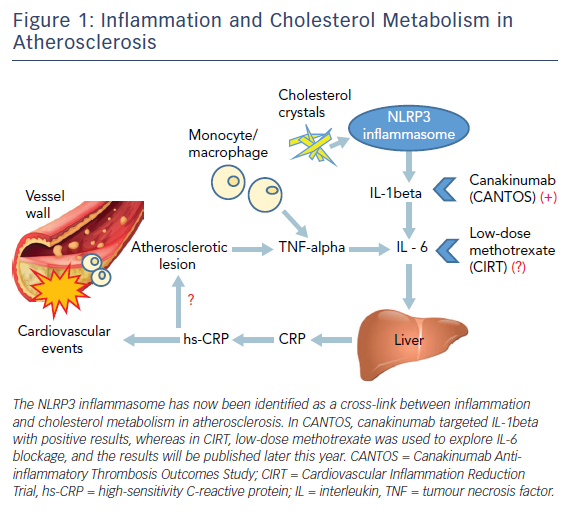
Recently, another factor within atherosclerotic plaques, the cholesterol crystal, has been identified as the predominant endogenous danger signal that initiates an inflammatory response via stimulation of the caspase-1-activating NOD-like receptor pyrin domain-containing-3 (NLRP3) inflammasome.9 As a result of retention of lipoproteins in the vessel wall, cholesterol accumulation may result in the formation of cholesterol crystals, which are taken up by macrophages and elicit an inflammatory reaction through the activation of the NLRP3 inflammasome, leading to an amplifying cascade of immune responses. Therefore, cholesterol crystals may be an initiating and/or an exacerbating factor in atherosclerosis by inducing cell injury and apoptosis.9
The major function of NLRP3 is to sense phagocytosed material and relay the signal to caspase-1, resulting in proteolytic cleavage and secretion of interleukin (IL)-1beta (pro-interleukin) as bioactive IL-1beta and IL-18, ultimately leading to increased production of other downstream inflammatory cytokines.10 IL-1beta and IL-6, among other systemic inflammatory mediators such as TNF-alpha, are then released into the circulation, leading to hepatic production of C-reactive protein (CRP).11 Consequently, the NLRP3 inflammasome has now been identified as a cross-link between inflammation and cholesterol metabolism in atherosclerosis (Figure 1).12
Targeting Inflammation in CAD
Despite compelling data from studies in animals and humans, the final confirmation of the inflammatory hypothesis of atherosclerosis has remained elusive. Serum biomarkers of inflammation, such as high-sensitivity CRP (hs-CRP), were independently shown to predict the risk of CV disease in observational studies.13,14 In addition, as treatment with statins reduces the levels of both LDL cholesterol and CRP, with a concurrent reduction in the number of CV events, the idea of targeting inflammation as a way of reducing CAD mortality and morbidity has received strong support. Indeed, the role of hs-CRP in CV disease prevention became clearer after the publication of the results of the Justification for the Use of Statins in Primary Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) study in 2008.15
In the JUPITER study, the degree of CRP lowering following statin therapy was significant and the therapeutic benefit of this intervention independent of the lipid-lowering effect was also predicted.16 In addition, a pre-specified analysis showed that a lower number of CV events were observed in patients who achieved both very low LDL cholesterol (<1.81 mmol/l) and low hs-CRP (<1 mg/l) levels.
Recently, in secondary prevention IMProved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT), this relationship between CV events (CV death, major coronary event, or stroke) and LDL cholesterol and hs-CRP levels was analysed in patients randomly assigned to simvastatin monotherapy or a combination of simvastatin and ezetimibe. In the 15,179 individuals studied, simvastatin plus ezetimibe significantly increased the likelihood of reaching the pre-specified dual target of LDL cholesterol <1.81 mmol/l and hs-CRP <2 mg/l one month after randomisation. Those achieving both targets (39 %)had lower primary endpoint rates than those meeting neither target (14 %) (38.9 % versus 28.0 %; adjusted HR 0.73; 95 % CI [0.66–0.81]; p<0.001).17 Although the addition of ezetimibe increased the likelihood of target achievement, the specific choice of agent used to reach the target did not have any influence on the outcome.17
Interestingly, this approach (lower values for both LDL cholesterol and hs-CRP being desirable) seemed to work even at low LDL cholesterol levels. In IMPROVE-IT, where a significant number of patients had an on-treatment LDL cholesterol level of <1.3 mmol/l, the rates of the primary endpoint were decreased in all subgroups when a lower exploratory target of LDL cholesterol <1.3 mmol/l and hs-CRP <1 mg/l were applied. Therefore, a fundamental question remains about whether or not inflammation can still play a significant role in patients who achieve ‘ultra low’ LDL cholesterol levels such as ≤0.78 mmol/l when, for example, treated with proprotein convertase subtilisin/kexin type 9 inhibitors, which have been shown to be unable to modify hs-CRP levels.18
Independent of their LDL-lowering capacity, statins have also been shown to attenuate inflammation.19 However, despite their success in reducing inflammation, LDL cholesterol levels and CV events, the major issue of targeting inflammation remained unresolved. Statins did not provide a proof of inflammation causality in atherosclerosis. The only way of resolving this issue by was testing the inflammatory hypothesis of atherosclerosis, without reducing LDL cholesterol levels and directly randomising patients to be targeted for anti-inflammatory therapies.
CANTOS: the Eagerly Awaited Proof of Concept
Of the various pathways and inflammatory mediators that have been implicated in atherogenesis, cytokine IL-1 of the innate inflammatory response is considered to be a ‘master cytokine’ in local and systemic inflammations. It also seems to play a central role in the atherosclerotic process.20 Furthermore, blocking IL-1 activity has revealed a pathological role of this cytokine in a broad spectrum of diseases, including heart failure and diabetes.21,22
Other related polypeptides IL-1alpha, IL-1beta, and the IL-1 receptor antagonist (IL-1Ra) are constituents of the IL-1 family, and are predominantly synthesised by mononuclear phagocytes and endothelial cells in response to microbial or endogenous stimulus triggers such as cholesterol crystals.23
Classical risk factors, such as dyslipidemia, diabetes, smoking or hypertension, and circulating levels of IL-1beta have been associated with CAD, along with higher concentrations of IL-1beta and IL-1Ra in atherosclerotic coronary arteries compared with normal arteries.24 Similarly, NLRP3 inflammasome and downstream cytokine (IL-1beta and IL-18) levels were linked with the severity of CAD, and variations in their levels were seen in patients with acute MI. In addition, an increase in the plasma levels of IL-1beta and IL-18 was associated with CAD severity. These results revealed that the increased expression of NLRP3 and downstream cytokines might be reflected in CAD severity.25
This pathophysiology background reinforced by a number of studies supported the rationale for specifically targeting IL-1beta, because IL-1alpha may participate in the host defence.22 Neutralising IL-1beta antibodies has been shown to prolong drug efficacy by several weeks after the cessation of therapy. This implies that only a low dose is required to treat an IL-1beta-mediated disease, which is an emerging and optimal strategy for chronic diseases such as CAD.23
CANTOS was the first large randomised controlled clinical study to examine the use of canakinumab, a fully human anti-IL-1beta monoclonal antibody, in the prevention of CV events in subjects with prior MI and elevated levels of hs-CRP, despite undergoing the usual therapy including high statin doses, and a baseline LDL cholesterol level of 2.12 mmol/l. The study included 10,061 subjects and was shown to reduce the primary endpoint of MI, stroke, or CV death by 15 % with a dosage of either 150 mg or 300 mg every three months. No change in LDL cholesterol was seen, whereas large concomitant reductions in hs-CRP and IL-6 were produced.26 The secondary endpoint, which included urgent revascularisation, revealed an even more significant result, with a 17 % relative risk reduction over a median follow up of 3.7 years.26 In addition, demonstrating the importance of inflammation in multiple systemic disorders, the inhibition of IL-1beta in the CANTOS also reduced incident lung cancer and lung cancer mortality by more than half in a dose-dependent manner.27
It is important to note that in CANTOS, among the included patients who represent a high-risk group characterised by a median hs-CRP of 4.1 mg/l, 40 % had diabetes, there was a significant number of smokers, and four out of five had already undergone revascularisation. Thus, the incidence rate for CV events in the placebo group was roughly two times higher than contemporary secondary prevention studies.
Most importantly, the effect on CV endpoints was strongest in those who were identified as ‘responders’ based on the fact that their achieved hs-CRP levels during the study were below the median hs-CRP in the overall population, and in this group the relative risk reduction amounted to 27 % (p<0.001). Among these robust responders, CV mortality and all-cause mortality were reduced. In addition to these clinically relevant reductions in CV outcomes, other pro-inflammatory diseases, such as arthritis, osteoarthritis, and gout, were significantly reduced. Nevertheless, there was no significant difference in all-cause mortality (HR for all canakinumab doses versus placebo 0.94; 95 % CI [0.83–1.06]; p=0.310). The fact that may limit the use of this drug in ischaemic heart disease is that canakinumab was associated with a higher incidence of fatal infection, small in proportion but significant.
In conclusion, the anti-inflammatory therapy of targeting the IL-1beta innate immunity pathway with canakinumab at a dosage of 150 mg every three months significantly reduced the recurrence of CV events in patients with elevated hs-CRP, compared with placebo. This beneficial effect was independent of any lowering effects on cholesterol levels.
Interestingly, in a recently published secondary analysis, individuals allocated to canakinumab who achieved hs-CRP concentrations <2 mg/l had a 25 % reduction in major adverse cardiac events (multivariable adjusted HR 0.75; 95 % CI [0.66–0.85]; p<0.0001), whereas no significant benefit was observed among those with on-treatment hs-CRP concentrations ≥2 mg/l (HR 0.90; 95 % CI [0.79–1.02]; p=0.11). Similarly, canakinumab treated patients who achieved on-treatment hs-CRP concentrations <2 mg/l, showed a significant reduction in both CV mortality (HR 0.69; 95 % CI [0.56–0.85]; p=0.0004) and all-cause mortality (HR 0.69, 95 % CI [0.58–0.81], p<0.0001), whereas no significant reduction in these endpoints was observed among those treated with canakinumab who achieved hs-CRP concentrations of ≥2 mg/l.28
By confirming the inflammatory hypothesis of atherosclerosis, CANTOS is a possible game changer. However, whether canakinumab can be included among the drugs to be used in CAD patients will depend on additional studies. In the meantime, secondary prevention patients with elevated CRP who clearly respond to a single dose of canakinumab by significantly reducing their hs-CRP levels, seem to be candidates for receiving the treatment.28
Related to this, observational studies in patients with rheumatoid arthritis (RA) support the idea that the immune modulator drug methotrexate has a favourable impact by attenuating the systemic inflammation and decreasing CV events.29 Moreover, a systematic review of the effect of methotrexate on CV disease in patients with RA concluded that its use was associated with a reduced risk of CV events, suggesting that methotrexate improves concomitant atherosclerosis in such patients.30 This favourable effect has been corroborated in a recent meta-analysis, where methotrexate was associated with a 21 % (95 % CI [0.73–0.87]; p<0.001) lower risk for total CV disease and an 18 % (95 % CI [0.71–0.96]; p=0.01) lower risk for MI.31
The Cardiovascular Inflammation Reduction Trial (CIRT) used methotrexate in patients with chronic atherosclerosis and either diabetes or metabolic syndrome, who were being randomised to low-dosage methotrexate, 15–20 mg/week or placebo. The trial was recently stopped after 4,786 patients of the planned 7,000 patients had been enrolled.32 The sponsor of the study, the National Heart, Lung, and Blood Institute, stated that there were no substantive safety concerns but the trial had accrued enough data to answer the main question of the study, and these results will be presented at the American Heart Association meeting in November 2018.
Colchicine, a classic anti-inflammatory drug used to treat gout, is also being tested for CV protection and represents a potentially useful agent for inflammation in atherosclerosis. Colchicine reduces the downstream production of hs-CRP, and recently a newly discovered mechanism has been described. Colchicine appears to block the crystal-induced activation of the NLRP3 inflammasome, thereby decreasing secretion of the pro-inflammatory cytokines IL-1beta and IL-18.33 In a preliminary open-label trial of 532 patients, low-dose colchicine showed promise for secondary prevention and, interestingly, the active intervention significantly reduced the primary endpoint of recurrent ACS, cardiac arrest or non-embolic stroke (HR 0.33; 95 % CI [0.18–0.59] p=0.001). Following on from CIRT, two double-blind, placebo-controlled trials are on-going; Low Dose Colchicine for secondary prevention in table Coronary Heart Disease (LoDoCo2) and Colchicine Cardiovascular Outcomes Trial (COLCOT) in patients after ACS.
Canakinumab is currently the only anti-inflammatory agent that has been proven to reduce CV events in patients with elevated markers of inflammation. At the time of writing, we are awaiting the results of other trials with alternative agents, including low dose methotrexate and colchicine.
Among these, individuals who respond with a robust reduction in hs-CRP level -reaching <2 mg/L after an initial canakinumab dose-, would be the ideal candidates for this anti-inflammatory approach since a significant risk reduction in both total and cardiovascular mortality was observed in this specific group of patients in CANTOS study.