“The tragedy of science is the slaying of a beautiful hypothesis by ugly facts.”
– Thomas Huxley
The 2016 AF European guidelines specifies that the rhythm control strategy – irrespective of method – exclusively addresses the control of symptoms and improvement of quality of life, autonomy and social functioning.1 The exception, obviously, is the vital indication of acute rhythm restoration in the case of the haemodynamically compromised patient.
The guidelines statement may generate puzzling reactions. First, despite scientific evidence showing no difference in outcomes between rate and rhythm strategies, many practitioners believe that maintaining sinus rhythm (SR) improves outcomes in AF patients. Second, the false impression that SR and AF are equivalent in terms of heart function and outcome may be deduced. The subanalysis from the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study clearly demonstrated that preservation of SR is accompanied by better survival (risk reduction is approximately 46%).2 However, antiarrhythmic drugs (AAD) used for SR preservation induce an almost equivalent increase in mortality, so it is not the preservation of the SR under question but the tools available to accomplish this target.
The recent Catheter Ablation Versus Medical Rate Control in Atrial Fibrillation and Systolic Dysfunction (CAMERA-MRI) study emphasised the importance of SR for optimising LVF in patients with systolic dysfunction of uncertain aetiology.3 In this study, the left ventricular ejection fraction (LVEF) improved significantly at 6 months in patients who underwent catheter ablation of AF compared with patients treated with rate control therapy (mean difference in ejection fraction 14%; 95% CI [8.5–19.5%]). However, this important study has shown that the improvement was obtained mainly in patients with minimal ventricular fibrotic myocardial remodelling, as demonstrated by late gadolinium enhancement during MRI examination. The study underlines the importance of the morphologic modification of the substrate such that the benefit of maintaining SR should be extended beyond symptom control.
There are important limitations regarding pharmacological restoration and maintenance of SR in patients with AF. The efficacy of AAD to convert AF and maintain SR is low. The real efficacy of AAD in conversion of AF is further biased by the fact that more than half of AF episodes convert spontaneously in 24 hours.4,5 The efficacy of AAD in maintaining SR is also modest – ranging from 19% to 60%, depending on the type of drug – and clinically efficient AAD therapy is reflected more in reduction of the AF burden (number and symptoms of AF episodes) than elimination of the arrhythmia.6 Moreover, there are important safety issues with AAD, including the high rate of proarrhythmia and drug withdrawal; sotalol accounts for the greatest mortality rate when compared with dronedarone or flecainide.6–8
However, it should be emphasised that the interpretation of drug safety is dependent on study design. Good examples are A Trial With Dronedarone to Prevent Hospitalization or Death in Patients With Atrial Fibrillation (ATHENA) and Permanent Atrial fibriLLAtion Outcome Study Using Dronedarone on Top of Standard Therapy (PALLAS), both with dronedarone in AF patients.9–11 ATHENA included high-risk patients with paroxysmal or persistent AF and demonstrated a decrease in cardiovascular hospitalisations and death, while PALLAS included ill patients with permanent AF and showed increased rates of heart failure (HF), stroke and death.
There are important safety concerns regarding the use of AAD in patients with concomitant structural diseases, which considerably limits the available adequate pharmacological solutions, i.e. either dronedarone, sotalol or amiodarone for patients with significant left ventricular hypertrophy and coronary artery disease, or only amiodarone for HF patients. This is concordant with the limited awareness of optimal drug therapeutic solutions; over one-third of cardiologists admit insufficient knowledge to assess the indication for rhythm control.12 The aforementioned limitations translate into practical use of AAD that is inconsistent with guidelines, excessive use of amiodarone despite well-known extracardiac side-effects and unexpectedly high rates of drug discontinuation.13,14
In the early 1990s, the Sicilian Gambit investigators created a complex concept for understanding the electrophysiological and clinical requirement for efficient and safe AAD use. Unfortunately this was considered “clinically unwieldy and... never fully accepted”.15,16 It became evident that the classical ADD-based therapy is empirical and drug/electrophysiological action centred, not arrhythmia and patient focused.17 Also, the classical AAD combine positive effects on arrhythmia with negative ones; for example, Class III Singh-Vaughan-Williams AAD have antifibrillatory properties by opposing the shortening of the action potential duration, but this is blunted by the increase in the chance of triggered activity. None of the classical AAD specifically address the vulnerable parameter of AF, as defined by the Sicilian Gambit investigators. However, the gap between available AAD and the expectations of pharmacological rhythm control in AF was partly overshadowed by the increasing interest in interventional electrophysiology and its tremendous development.
AF Ablation: The Last Frontier?
Catheter ablation techniques have diversified and refined during the last decade. Classical radiofrequency energy source and newer cryoablation source demonstrated similar success rates.18 Overall, AF ablation is accompanied by freedom from AF recurrences of more than 60% (without AAD) in the first year.19 However, this benefit is significantly blunted during longer follow up, reaching 40% at 5 years.20,21
The most important aspect of the superiority of AF ablation when compared with AAD therapy is conferred by symptom control and improvement in quality of life and functional capacity. There are no convincing data regarding the general impact of AF ablation on hard endpoints such as mortality or major adverse cardiac effects. The results and rates of success of AF ablation studies are heterogeneous because important differences exist between specific populations with AF. Persistent and permanent forms of AF are less susceptible to consistent effects because of important cardiac remodelling. The success of a single procedure in persistent AF is as low as 43%; however, repeated procedures and novel sophisticated techniques – complex fractionated electrograms, linear ablation in the left atrium, rotor mapping and ablation or substrate modification – can improve the outcome.22,23
Safety issues are linked to procedure complexity, patients’ associated comorbidities and experience of ablation centres.24 Although there is a temporal decrease in complication rates, the number of adverse events remains high even in experienced, high-volume centres, with elderly and HF patients being more exposed.25 A recent meta-analysis/meta-regression investigation showed that AF ablation is superior to AAD therapy in terms of AF recurrences but non-superior in terms of adverse effects.26 Moreover, the authors noted a regression in efficacy since 2011.
The position of the guidelines regarding AF ablation has been reconsidered and upgraded as first alternative of rhythm control therapy in all forms of symptomatic AF (grade 2a for paroxysmal and persistent AF and 2b for long-standing AF).27 However, the place of AF ablation as first-line therapy versus AAD therapy is still a subject of debate and insights into this subject will be offered by the Early Treatment of Atrial fibrillation for Stroke Prevention Trial (EAST).28,29
The success rate of AF ablation is significantly increased by concomitant use of AAD, but the residual recurrence risk remains high over time.19 Short-term AAD therapy is also used to avoid early AF recurrences after catheter ablation; however, the benefit is still debatable.30–33 Long-term use of AAD therapy post-ablation remains an important tool for preserving SR in patients using previously ineffective AAD.34 Several reasons indicate that HF patients are a particular target for AF ablation. More than 30% of HF patients have AF. Traditional drug therapy with beta-blockers was not associated with a significant reduction in mortality in patients with concomitant AF and systolic HF.35 Data have shown that AF ablation reduced the risk of cardiac hospitalisation and recurrent atrial arrhythmia in subjects with HF and in subjects without HF; however, the reduction in all-cause mortality was noticed only in subjects with HF.36
Two contemporary studies have raised enthusiasm and hope but also scepticism. The Catheter Ablation for Atrial Fibrillation with Heart Failure (CASTLE-AF) study included 394 patients – from 3,013 screened – with HF with reduced ejection fraction (ejection fraction <35%) and symptomatic paroxysmal or persistent AF.37 Patients were randomised for ablation (179 admitted, 21 excluded; patients underwent isolation of pulmonary veins, additional lesion lines and repeated procedure after blanking period, all being permitted) or conventional therapy (184 patients admitted, 13 excluded; 70% of them receiving a rate control strategy, AAD being discouraged and 30% receiving rhythm control strategy, mainly based on amiodarone). A crossover of 26 patients in the ablation group and 18 patients in the conventional therapy group was reported. Mean follow up was 37.8 months and the primary endpoint was all-cause mortality and hospital admission for worsening HF. The results showed an important reduction in the primary composite endpoint (38% risk reduction) and in the secondary endpoint of all-cause mortality (47% relative risk reduction). The AF burden was also significantly decreased in the ablation group. The benefit on mortality only emerged after 36 months, pointing to a sufficiently long time to observe the benefit in ablation trials.
Despite the impressive results, apparent study limitations and commentaries invite moderation when interpreting the findings.38 The study group included mostly young patients, almost exclusively male, with less severe disease (New York Heart Association class I and II). On the contrary, in the conventional therapy group patients had a trend for more severe disease (more diabetes, more ischaemic, more taking digoxin). The results could also be biased by the fact that 13% of patients in the ablation group were lost to follow up, compared with only 5% in the conventional group. A query regarding the patient selection for this study is also raised by the fact that the number of screened patients was 10 times higher than that of included patients, with one patient included per site and per year. When looking at subgroup analysis, the benefit does not translate to females, patients aged >65 years, those with LVEF <25% or those with previous ventricular tachycardia/VF. The number of patients with missing or excluded data or events in the ablation group was almost double that of the conventional group, which also influences the interpretation of results. There are no detailed data about how HF was treated according to modern guidelines in the two groups, or about how symptomatic AF was defined to exclude symptoms caused by HF. Finally, the number of events during the study was 32% less than prespecified by the power calculation.
The long-awaited Catheter ABlation versus ANtiarrhythmic Drug Therapy in Atrial Fibrillation (CABANA) study included 2,204 patients with new-onset or untreated AF and increased cardiovascular risk randomly assigned to either catheter ablation or drug therapy.39 This study had a primary composite endpoint of all-cause mortality, disabling stroke, serious bleeding and cardiac arrest. Initially, the primary endpoint was all-cause mortality but because of the lower-than-expected number of events and inclusion rate, it was changed and the sample size was reduced to 2200 patients. The alternative design adopted was characterized by Milton Packer as “the terrifying power of self-deception”.40
Despite this change, the study failed to demonstrate any benefit in intention-to-treat analysis of the primary endpoint, or of all-cause mortality. There was a significant reduction in the combined endpoint of cardiovascular death and hospitalisations. However, this could be better explained by the decrease in readmissions for AF.41 This study also included relatively young patients of who only 25% had previously diagnosed HF. Bleeding contributed to more than 40% of the composite endpoint – probably to the same extent in both groups – and all-cause mortality was low.42 Had this been a trial for a new drug, the on-treatment analyses would probably have been rejected as a result of all their sources of bias.41 Despite significant limitations, both studies are important to clinical practice; they do not change the actual guidelines but reinforce them. They confirm the safety and the efficacy of ablation and warrant the use of this procedure in the early stages of HF and in patients where at least one AAD has failed.
Complexity of AF: The Secrets of Present Failure and the Future Success of Rhythm Control
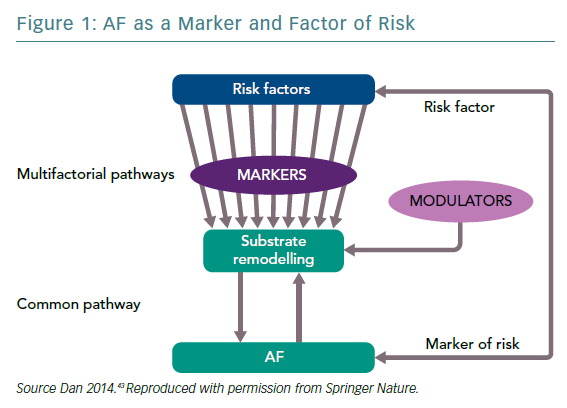
The existing limitations of the rhythm control strategy in AF – irrespective of the methodology – are a result of the complexity of arrhythmia. AF is a multifactorial arrhythmic syndrome with a common electrical phenotype. It is a marker – a witness of the disease and/or its severity – and a risk factor for cardiovascular disease (CVD) with causal implications. As such, rhythm control as a part of the management of AF considered as a risk factor implies prevention of CVD or its progression (Figure 1).43
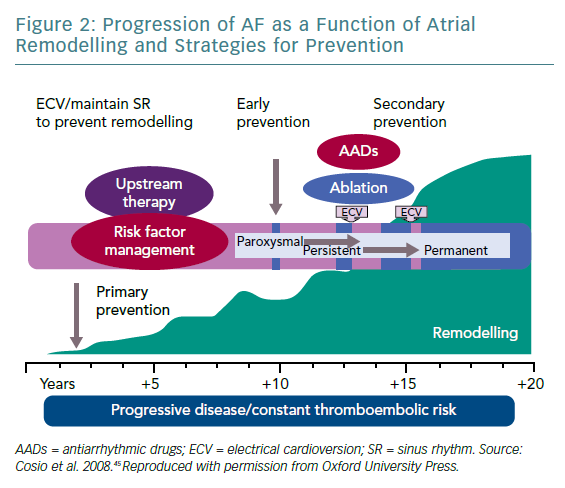
The key element for AF initiation and perpetuation is represented by substrate remodelling under the influence of traditional risk factors and under the influence of the genetic susceptibility (the two-hit hypothesis).44 The remodelling process involves electrical substrate (ion channels), functional substrate, morphological substrate (fibrosis) and the intracellular calcium handling (responsible for triggered and ectopic activity). Substrate remodelling and triggered activity – essential for AF initiation and perpetuation – are the vulnerable targets for the efficient rhythm control. 'AF begets AF' is a phrase pointing to AF-induced fibrotic remodelling. However, the remodelling process is more complex including, as previously shown, the contribution of genetic factors, age and associated diseases or risk factors. The success of strategies aimed at maintaining the SR is highly dependent on timely intervention and the degree of substrate modification (Figure 2).45 The Routine Versus Aggressive Upstream Rhythm Control for Prevention of Early Atrial Fibrillation in Heart Failure (RACE 3) study demonstrated that targeted therapy of underlying diseases improves SR maintenance in patients with persistent AF.46
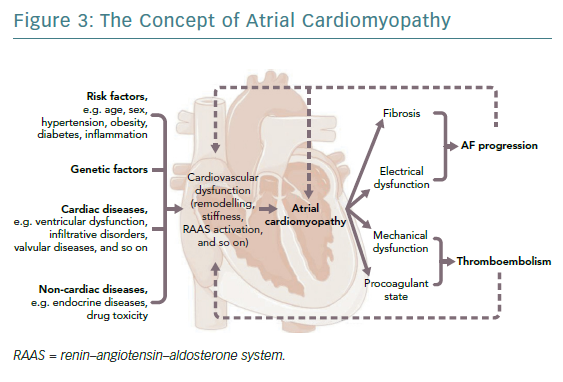
The fibrotic remodelling may have mechanisms independent of AF and may precede it.47,48 The generic term ‘atrial cardiomyopathy’ was created to define the entire spectrum of processes involved in atrial remodelling including electrical, functional, morphologic and procoagulant dysfunction to which AF is associated (Figure 3).49,50
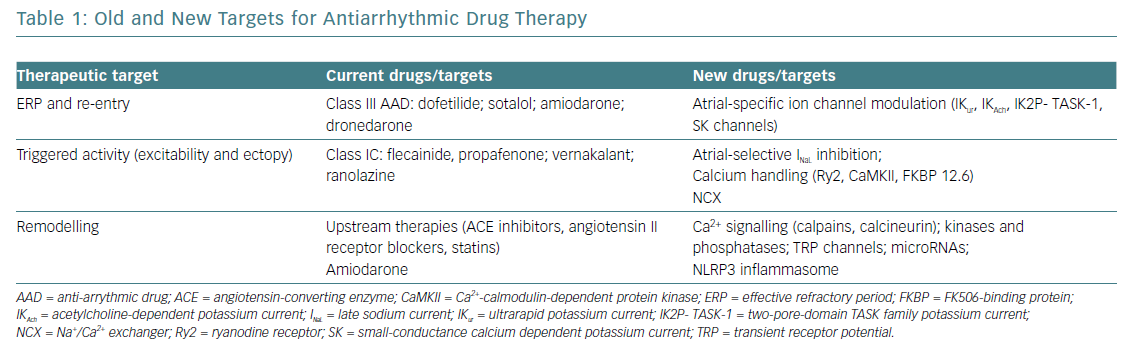
Both interventional and pharmacological therapy need to consider the acquired progress in understanding AF mechanisms. New targets for AAD may be represented by specific atrial currents, remodelled in AF, such as the ultrarapid potassium current (IKur), the acetylcholine-dependent potassium current (IKAch), the two-pore-domain TASK family potassium currents (IK2P) or the small-conductance calcium dependent potassium currents (SK).17 Obviously, targeting specific atrial currents will increase AAD safety, diminishing the risk of ventricular proarrhythmia.
Another important target for modern AAD is represented by the components of altered intracellular calcium handling (ryanodine receptors Ry2, Ca2+-calmodulin-dependent protein kinase [CaMKII] or calstabin [FKBP12.6]). Other possible targets – non-coding microRNAs and, unexpectedly, components of the inflammatory chain, such as the NLRP3 inflammasome system – recently demonstrated contribution to atrial remodelling. Unfortunately, because of social and economic reasons the gaps between what we can do and what we should do remain important, especially concerning pharmacological therapy.51 The perspective on future AAD is summarised in Table 1.52
In clinical practice, pharmacological AAD therapy and ablation are often viewed simplistically as competitors. However, it should be emphasised that ablation and pharmacological therapy are complementary tools – both far from ideal at this moment – and they should develop in parallel with the new paradigm of AF. As Hamlet said: “There are more things in heaven and earth, Horatio, than are dreamt of in your philosophy”.53