Renal sympathetic denervation (RDN) has been introduced as a novel approach to treat patients with so-called resistant hypertension.1–3 The first randomised clinical trial showed an impressive 32/12 mmHg fall in blood pressure after six months in the intervention group (n=52) compared to the control group (n=54). However, with the recent publication of the Symplicity HTN-3 study in the US4 the world has become in doubt whether RDN lowers blood pressure. An editor of distinguished journal5 published his reflections and stated that the Symplicity HTN-3 results came as a shock to the world; a single but large and properly designed prospective randomised clinical trial could on its own neutralise hundreds of mostly observational studies, case reports and other enthusiastic publications emphasising the amazing effect of RDN, not only in patients with resistant hypertension, but also in a host of other diseases and conditions.
The initial enthusiasm followed by the setback of RDN can probably be summarised by a handful of explanations: 1) The role of the sympathetic system in the pathophysiology of hypertension is substantiated by a wealth of experimental and clinical arguments.6–12 On this background, enthusiasm surged when an intervention in this system seemed to drastically lower blood pressure; 2) Market-driven industry took control and exerted unprecedented influence on the medical community; 3) Subsequently, pitfalls in apparent treatmentresistant hypertensive patients, which are simple but well-known for decades, were suddenly forgotten including well described phenomena such as the placebo effect, regression to the mean, poor drug adherence13–15 and the Hawthorne effect.
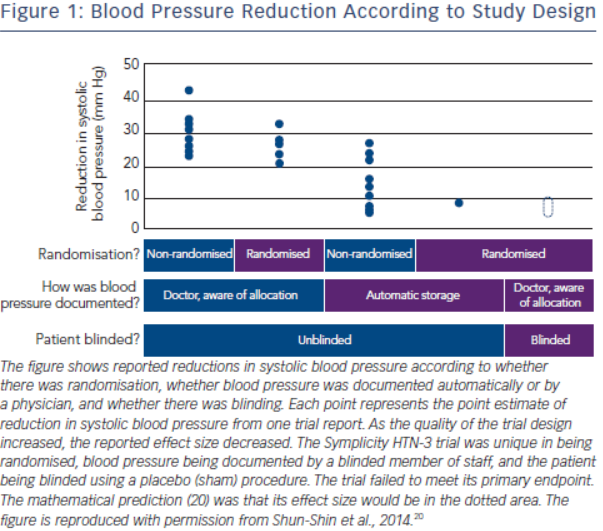
The history of the rise and decline of RDN deserves a more in-depth analysis. The first and for long the only prospective randomised clinical trial in this field, the Symplicity HTN-2 study2, was monitored by Ardian (Medtronic) who collected and processed the data.2 Usually, when such a task is given to industry, all measures are taken to secure confidence and trials are prospective, randomised and double-blinded.16–19 However, in this case, everything was open, making the trial particularly vulnerable to patients, physicians and sponsor-related biases.19 As indicated by Shun-Shin et al. in a recent editorial,20 “measurement of a noisy variable by unblinded optimistic staff is a known recipe for calamitous exaggeration”. This is illustrated in Figure 1. It is unfortunate that selection of patients enrolled in Symplicity HTN-2 and evaluation of efficacy were based on office rather than ambulatory blood pressure (ABPM), which is state-ofthe art,21 particularly in resistant hypertension.22 ABPM reduces observer bias and measurement error, minimises the white-coat effect and has greater reproducibility, and therefore provides a better estimate of a patient’s usual blood pressure and cardiovascular prognosis.23,24 Notwithstanding the well-known, major contribution of poor drug adherence to apparently resistant hypertension13–15,25–27, drug adherence was not monitored, either at baseline or during follow-up. This made the study vulnerable to the Hawthorne effect, i.e. patients changing behaviour – in this case starting taking their drugs as prescribed – in response to the intervention and massive attention devoted to them. The lack of blood pressure decrease in the control group also raised concerns. One would indeed suspect that patients in the control group had not taken their medications properly, in order to keep their blood pressure at a higher level that made them eligible for cross-over to RDN group.28,29 Finally, placebo effect and regression to the mean must also be taken into account. Noteworthy, the placebo effect is small by using ambulatory blood pressures21,30; however ambulatory blood pressures remain as sensitive to the Hawthorne effect as office blood pressure.
Despite the major limitations and potential biases of Symplicity HTN-2, a small open study with suboptimal design including only 106 patients followed up for six months, RDN was adopted in hundreds of centres worldwide. Medtronic Inc® (Minneapolis, Minnesota) paid $800 million to purchase Ardian® (Mountain View, California), the company that had developed the technology5, and more than ten companies developed their own RDN systems, five of which obtained the CE mark. The procedure was quickly reimbursed in Germany, and later on in Switzerland, Sweden and the Netherlands. While RDN remained an investigational procedure in the US, at least 8,00031, possibly 15,000 to 20,000 procedures were performed in Europe and in the rest of the world in less than four years, most of them using the Ardian-Medtronic® catheter. It may be hypothesised that the massive incomes generated by selling the Symplicity catheter to enthusiastic Europeans contributed to the expenses of the Symplicity HTN-3 study4, required by the Food and Drug Administration (FDA) before approval of RDN in the US.
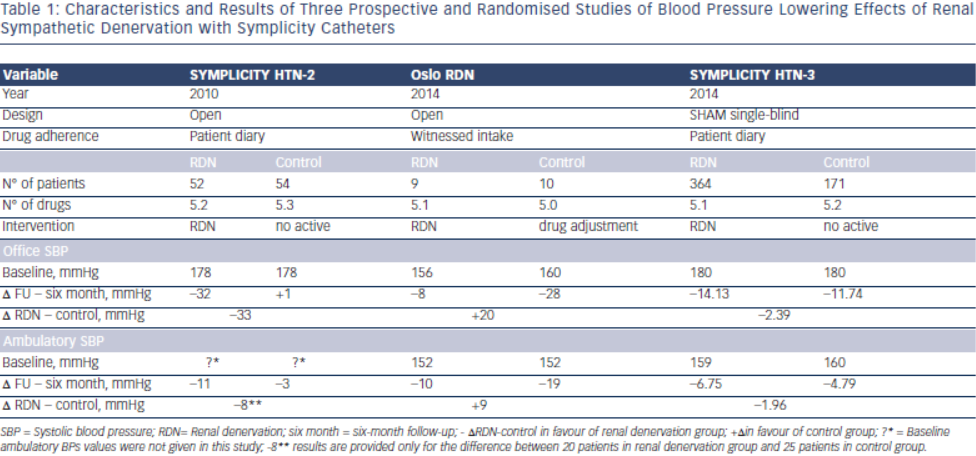
In Symplicity HTN-34, blinding of patients through the use of a sham procedure and wider use of ambulatory blood pressure measurement balanced and limited the differential impact of the Hawthorne, white coat, placebo and regression to the mean effects in both treatment arms. This disclosed to the world the true size of blood pressure decrease attributable to RDN, at least in patients meeting the Symplicity criteria; it was less than 3 mmHg systolic based on ambulatory blood pressure monitoring (Table 1). Sham-procedure is however not feasible in clinical practice but was required by FDA in the Symplicity HTN-3 Study to overcome all the pitfalls in hypertension research mentioned above in order to investigate whether RDN has a true blood pressure lowering effect and the procedure may be characterised as “evidence based medicine”. Taken together with another four prospective and randomised clinical trials published or presented in 2014, discussed below, RDN as of today obviously does not fulfill these criteria and should not be used outside research protocols.
For all aforementioned reasons, and in view of the complexity and multifactorial character of hypertension, the failure of RDN to normalise or substantially reduce blood pressure in all patients with apparently resistant hypertension was a reasonable working hypothesis for us, even before the Medtronic announcement that Symplicity HTN-3 had failed to meet its primary endpoint (http://www.tctmd.com/show. aspx?id=123265 ). We32–34 and others19,28 had predicted that the true effect of RDN might have been overestimated and may considerably shrink in properly designed studies.19,29 In particular, in preliminary analysis of the European Network COordinating research on Renal Denervation (ENCOReD) network,35 we were struck by the imbalance between the 17.6 mmHg decreases in office blood pressure, vs only 5.9 mmHg for 24-h ambulatory blood pressure.
The ENCOReD site in Oslo, with longstanding traditions for randomised research in hypertension36, applied a simple and practical way to deal with pitfalls in the recruitment of patients with resistant hypertension. After extensively ruling out secondary hypertension, and improving drug treatment in the run-in phase, patients had to qualify for the RDN protocols by having elevated daytime ambulatory blood pressures after witnessed oral intake of their prescribed blood pressure medication.33 This was a convenient way to identify the true treatment-resistant hypertensive patients and to exclude patients with white coat hypertension or those non-adherent patients whose blood pressure normalised after witnessed drug intake. Meanwhile a centre in Germany37 published a small but well documented series of patients whose blood pressure remained unchanged after RDN. We were thus not surprised when the Oslo activity found no change in either office or ambulatory blood pressures following RDN, first in an open series of six patients33 and later followed by a randomised study, the Oslo-RDN trial.38 Patients who were randomly assigned to further improvement of drug treatment guided by noninvasive haemodynamic monitoring had normalised blood pressures. In contrast, patients exposed to RDN experienced only a small and probably partly placebo-induced fall in office and ambulatory blood pressures (see Table 1). The decreases averaged 20 mmHg more for office and 9 mmHg more for ambulatory systolic blood pressure in the haemodynamically guided drug treatment group compared to the RDN group.
In the absence of solid evidence of efficacy, how can we explain the uncontrolled deployment of RDN in Europe and worldwide (with the notable exception of the US where RDN remained an investigational procedure)? Of course, publications of the Symplicity studies and of multiple observational studies, and enthusiastic reports, editorials and reviews1–3,39,40 had a substantial impact, and the lack of strict rules for introduction of device-based therapies in Europe facilitated the large-scale implementation of the technique. However, this phenomenon would have remained limited without the huge promotion by device-producing industry. Medical journals were swamped by reviews and meta-analyses showing the powerful blood pressure lowering effects as recorded in observational studies and in the single available randomised study, Symplicity HTN-2. Comments pointing out the defects and inconsistencies in such meta-analysis encountered great delay in getting published.41 Many never questioned whether RDN should be implemented, but when it should start in an institution. By all means, the purpose was to disseminate the enthusiasm for RDN from the technically-oriented invasive radiologists and cardiologists who usually had little interest or experience in the treatment of hypertension to the “hypertension establishment.” The European Society of Hypertension issued specific guidelines,42,43 but maintained reservations that more data was needed, and eventually it had to be proven that RDN would lower morbidity and mortality before being generally accepted in the treatment of true or apparent treatment- resistant hypertension.
Unfortunately, the most enthusiastic proponents of RDN do not seem to have fully accepted the lessons of Symplicity HTN-3. In the aftermath of Symplicity HTN-3, a campaign has been set up to criticise the study because of including inexperienced investigators, who did not appropriately document the delivery of ablation energy in the renal arteries, and enrolling too many African American patients who improved their drug adherence in the course of the trial.31,44 Symplicity HTN-3 may have had its weaknesses, however, no subgroup analysis was statistically significant4 and all the hypotheses explaining the failure of Symplicity HTN-3 by showing a difference in blood pressure were post hoc and speculations. Besides, also this study was overseen by procedure experts (proctors) from the sponsoring company, namely Medtronic. Furthermore, the Symplicity HTN-3 results are diluted by non-scientific comparisons with the Medtronic® registry45 which is hampered by all the weaknesses touched upon in this commentary, and even more as it is a pure industry-ran activity. Finally, while RDN will not become available in the US, and ongoing research in Asia was stopped, Medtronic and other companies continued making their catheters available for clinical use in Europe and did not restrain from heavily promoting the technique, for example at the Euro PCR conference in Paris in May 2014 (www.medscape.com/author/shelley-wood).
One-year results of Symplicity HTN-34 were presented at the European Society of Cardiology (ESC) meeting in Barcelona in end of August 2014. Following the six-month evaluation all subjects and clinicians were unblinded and antihypertensive medications changes were allowed. Of the 171 original sham control group subjects, 101 who still fulfilled all of the baseline inclusion and exclusion criteria, and agreed to receive the procedure, crossed over to RDN. The remaining 70 sham control subjects did not cross over and were not treated with RDN. At one year post-procedure, both office and 24 hour mean ambulatory systolic blood pressure continued to demonstrate a decrease in the original RDN group. Similarly, the crossover group showed blood pressure reductions six months following RDN (Δ systolic blood pressure: 17.7 mm Hg, P<0.001). The non-crossover group also had large reductions in office pressure one year post randomisation (Δ systolic blood pressure: 21.4 mm Hg, P<0.001) (http://congress365. escardio.org/). In the summary session of the ESC meeting the keynote speaker (Bryan Williams, London, U.K.) on September 3 pointed out the uncertain future of RDN based on the recent randomised studies performed in Oslo38 and in the US.4
The key message of Symplicity HTN-34 is simple and we should be wise enough to accept it: the true overall benefit of RDN on systolic blood pressure is modest, <3 mmHg, without evidence of a favourable impact on morbidity-mortality so far. The results of three other recent rigorously designed randomised controlled trials, Oslo RDN38 (see Table 1), DENER-HTN46, and PRAGUE-1547 including a smaller number of well-trained operators are in line with those of Symplicity HTN-34, and confirm that the failure of RDN to achieve superiority over medical treatment in the latter cannot be merely explained by inclusion of a high proportion of African Americans or insufficient degree of renal nerve ablation. Along the same lines, a fourth randomised shamcontrolled study performed in mild resistant hypertension (daytime systolic blood pressure between 135 and 149 mmHg and/or diastolic blood pressure between 90 and 94 mmHg on ≥ 3 drugs classes including a diuretic), Symplicity Flex48, failed to show an advantage of RDN compared to drug treatment alone.
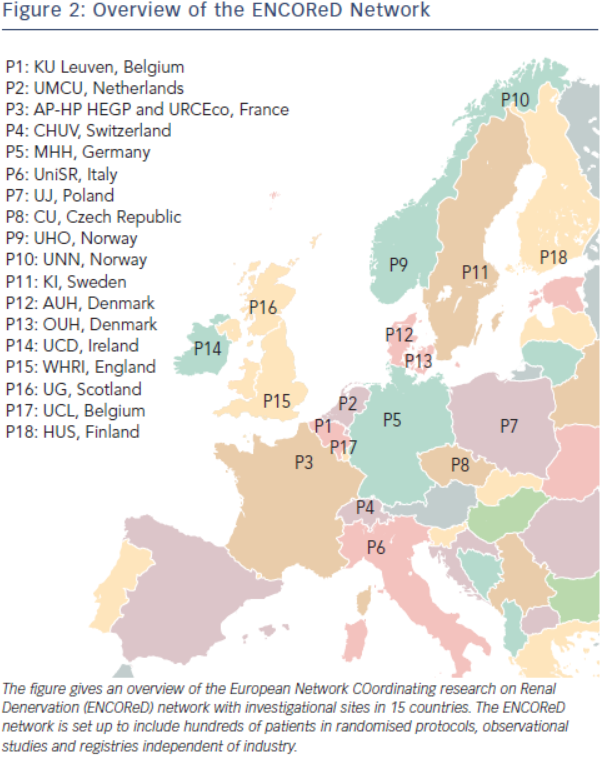
Does the failure of Symplicity HTN-3 mean the end of RDN? Not necessarily. Indeed, as already mentioned, RDN is based on a solid rationale substantiated by over fifty years of meticulous research of the sympathetic nervous system and its involvement in the pathophysiology of hypertension.6–12 Furthermore, it has been shown in cohorts recruited from the third (The effect of progressive sympathectomy on blood pressure, Walter Bradford Cannon 1931, http://www.ncbi.nlm.nih.gov/ pubmed/2204236) until the fifth decade of the last century49,50 that abdominal sympathectomy associated to splanchnicectomy is effective in the treatment of severe hypertension. Finally, many centres report major responses to RDN in a minority of patients.33,35,38 Accordingly, research should go on to find out the minority of patients who are true responders to RDN, and identify predictors of effective RDN. The ENCOReD network (see Figure 2) is set up to include hundreds of patients in randomised protocols, observational studies and registries independent of industry. Some early results35,51 from this joint effort have already been published and suggest that it may be worthwhile searching for potential predictors of response to RDN.
As of now, the modest overall benefits and high cost of RDN should be balanced with its potential risks. In particular, more than 20 cases of de novo renal artery stenosis have been reported after RDN,52 most of them after the announcement that Symplicity HTN-3 failed to meet its primary endpoint. RDN deserves further investigation but is not ready for clinical deployment and its use should be restricted to research protocols. Accordingly, in Germany, the insurances companies which were the first in Europe to reimburse the procedure have terminated their coverage, and even well-known proponents of the technique acknowledge that RDN “should be returned back to the academic arena”53 before further clinical deployment.
In a substantial proportion of patients, resistant hypertension may reflect resistance to taking medications rather than true treatment resistance.54 In this perspective, therapeutic drug monitoring may prove an effective strategy, not only for detecting poor drug adherence but also for improving blood pressure control. Indeed, when non-adherent patients are confronted with their low or undetectable drug levels and provided additional counselling to overcome barriers of adherence, blood pressure control improves considerably without intensification of therapy.55 Along the same lines, a recent analysis based on German data and life statistics showed that therapeutic drug monitoring is a cost-effective health care intervention in patients diagnosed with apparent drug-resistant hypertension, and this finding is valid for a wide range of patients, irrespective of age and sex.56 Even in truly resistant hypertensive patients with demonstrated drug adherence, blood pressure control may be achieved in a substantial proportion of patients by skilful drug treatment adjustment.33,38 While RDN deserves more in depth research, in the present state of knowledge, initiatives aiming at diagnosing and improving poor drug adherence56,57 and optimisation of drug treatment51 may prove much more cost-effective, both at the individual and public health level.