Myocardial revascularisation in patients with stable chronic angina is performed with the aim of reducing cardiovascular death, reducing myocardial infarction (MI) and relieving angina symptoms. However, contrary to expectations, modern therapy with percutaneous coronary intervention (PCI) has not had a significant impact on hard outcomes.1–5 Indeed, as also summarised in a recently published meta-analysis,6 PCI in stable angina patients does not reduce cardiovascular death or MI. Therefore, symptom relief remains the final rationale for suggesting our chronic angina patients undergo PCI.
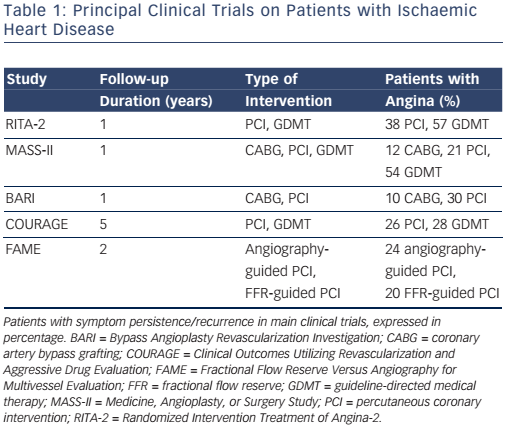
Major international guidelines for the management of chronic stable angina recommend a revascularisation procedure in patients with obstructive coronary artery disease (CAD), high risk features at noninvasive evaluation, and lack of symptom control under maximally tolerated medical therapy.7,8 Following revascularisation the majority of patients obtain symptom relief and improved quality of life. Nonetheless, angina symptoms and/or ischaemia may recur or persist in a significant patient subset.9,10 The reported rate for post-PCI angina and/or ischaemia is variable, and ranges from 15 % to more than 50 %.2–4,11–15 These findings have also been confirmed in more recently published studies that adopted modern therapeutic strategies (see Table 1).5,16,17 Besides reduced quality of life, symptom and/or ischaemia recurrence is associated with adverse cardiovascular events and increased healthcare costs.18,19 Therefore, identification and treatment of this patient population is warranted.
In this paper, we discuss potential pathophysiological mechanisms underlying post-PCI myocardial ischaemia and propose strategies to enhance their identification prior to the revascularisation procedure.
Pathophysiological Basis of Post-percutaneous Coronary Intervention Angina and Ischaemia
The traditional understanding of stable CAD is that of a disease causing exercise- and/or stress-related symptoms due to narrowing of ≥50 % in the left main coronary artery and/or of ≥70 % in ≥1 of the major arteries.7 For this reason, symptom recurrence following identification and removal of the coronary stenosis is regarded with great suspect. Indeed, post-PCI symptoms are frequently considered as either non-cardiac (i.e. of gastrointestinal or skeletal origin) or as non-ischaemic (i.e. stretch pain). Conversely, once the ischaemic nature is documented, it is attributed to a combination of procedurerelated factors (i.e. restenosis, incomplete revascularisation, atherosclerotic disease progression, epicardial coronary spasm, etc.), patient-related factors (left ventricular hypertrophy, aortic valve disease, etc.) or factors related to the methodology used to investigate persistent angina and/or ischaemia.20–23 However, in most series, the reported restenosis rate after stent implantation is <10 % and usually occurs 2–3 months after index PCI.24,25 Similarly, the extent to which incomplete revascularisation and disease progression contribute to persistent angina is much lower than the rate of persistent angina reported in the literature. In a registry study of 1,755 consecutive patients undergoing PCI, 26 % reported angina at 1-year follow-up. Patients with incomplete revascularisation reported a slightly higher angina rate (32 %). However, angina also recurred in 23 % of patients with complete revascularisation.26 In addition, following revascularisation, the rate of altered results at non-invasive testing despite no epicardial obstructions at coronary angiography is higher than in patients with initial angina diagnosis.27–29 Unfortunately, these data have not generated scientific curiosity. On the contrary, positive non-invasive test results are often considered ’false positive‘ and, for this reason, routine clinical assessment by means of non-invasive testing is not recommended within 2 years from index PCI and within 5 years from coronary bypass surgery in some countries.27,28,30–33
In order to control for all these confounding factors, we conducted an observational study on a highly selected, chronic angina patient population, undergoing complete PCI.17 Patients with valvular dysfunction, primary cardiomyopathy, or other conditions known to interfere with electrocardiographic interpretation, were excluded. We adopted an identical and serially repeated clinical evaluation with exercise stress testing and quality of life questionnaire at baseline and at early (1 month), medium (6 months) and long-term (12 months) time points after index PCI, thereby increasing the probability of obtaining highly reproducible and reliable stress test outcomes. Yet, of the 198 patients enrolled in the study, about one-third suffered angina with impaired quality of life and had a positive result at control followup visits with stress testing.
Microvascular Dysfunction as a Cause of Persistent Angina
Li et al. went a step further and hypothesised that microvascular dysfunction was the cause of post-PCI angina and/or ischaemia.16 They measured thermodilution-derived coronary flow reserve (CFR) and hyperaemic index of myocardial resistance (IMR) in 39 subjects with chest pain and 12 control subjects who were asymptomatic after PCI with angioplasty and stenting. Measurements were taken in the culprit vessel and in a non-culprit reference artery. No restenosis was documented in the study participants. Compared with the control group, patients with persistent symptoms had a higher resting IMR in both culprit and reference arteries. Persistent angina patients also had a higher hyperaemic IMR and a lower CFR, with 54 % of them displaying a CFR of <2.5. The authors concluded that coronary microvascular dysfunction was responsible for the post-PCI myocardial ischaemia in a significant part of these patients. The effect that PCI with stenting exerts on microvascular function is supported by conflicting evidence.16,34,35 Revascularisation is associated with microembolism and/or activation of platelets or microparticles, ischaemia-reperfusion injury, and exaggerated liberation of reactive oxygen species that lead to functional and/or structural dysfunction, and thus has been hypothesised to induce microvascular dysfunction.36 On the other hand, chronic low shear stress distal to the stenosis, with decreased nitric oxide activity37 or a low perfusion pressure that negatively influences microvascular remodelling and the capacity of maximal vasodilation after restoration of a normal basal coronary blood flow can also be the cause of persistent symptoms.38,39 Indeed, as documented in the study by Li and colleagues, a PCI-specific effect cannot fully explain the findings also observed in the reference vessels.16
Abnormal Coronary Vasomotion as a Cause of Persistent Angina
In another study, Ong and colleagues sought to determine the rate of abnormal coronary vasomotion to intracoronary acetylcholine (ACH) administration in 104 consecutive patients with persistent angina despite successful PCI.34 Focal or diffuse epicardial coronary diameter reduction of >75 % in any epicardial coronary artery segment and/or reproduction of patient’s symptoms/ electrocardiogram (EKG) abnormalities (microvascular spasm) were considered positive. Abnormal coronary vasomotion in response to ACH was found in 66 % of study participants, with 73 % of them displaying enhanced epicardial coronary vasomotion and 27 % displaying microvascular spasm.
Other Causes of Persistent Angina
Thus, when further investigated, an alternative cause for myocardial ischaemia (i.e. microvascular dysfunction, vasospasm) is identified in the majority of patients with post-PCI myocardial ischaemia. However, going back to the study by Li et al., only slightly more than 50 % of subjects with persistent symptoms had invasive measurements that would reach criteria for abnormal microcirculation, whereas the cause of persistent angina in the remaining patients remains undetermined.16 Similarly, only 66 % of patients displayed abnormal coronary vasomotion in the study by Ong et al.34 Other causes of persistent angina include incomplete revascularisation, endothelial dysfunction, inflammation, platelet dysfunction, coagulation abnormalities, and various combinations of them.40,41 All these factors are in line with the proposed multifactorial model for stable ischaemic heart disease (IHD), where obstructive CAD constitutes only one among a myriad of other factors.42
In line with considerations, the latest major international guidelines recognise that factors such as microvascular dysfunction and vasospasm can all induce myocardial ischaemia. However, according to the same guidelines, these factors are to be investigated only when obstructive CAD is excluded, as if epicardial stenosis conferred immunity versus the other mechanisms.
In fact, the lack of benefit of PCI continues to be related to the inaccurate assessment of epicardial coronary obstructions.43 As such, imaging modalities that assess epicardial coronary plaques have gained increasing relevance.
Fractional flow reserve (FFR) is the most popular modality used to assess the physiological effects of epicardial plaques. The Fractional Flow Reserve Versus Angiography for Multivessel Evaluation-2 (FAME-2) trial reported on the efficacy of FFR-guided PCI versus medical therapy in stable coronary disease.44 Patients with a coronary stenosis that produced a significant drop in pressure (FFR of 0.80 or less) in a major coronary artery were included in the study. Similar to the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial,14 the rate of revascularisation was the only outcome that significantly differed between treatment groups. In a registry-based study involving more than 7,000 patients referred for coronary angiography, those undergoing FFR measurements tended to have lower rates of death/MI (hazard ratio [HR]: 0.85, 95 % confidential interval [CI] [0.71–1.01]; p<0.060). However, among those patients undergoing FFR, the rate of MI was lower in those whom PCI was deferred. In particular, deferral of PCI guided by FFR was associated with a reduced rate of MI (HR: 0.46, 95 % CI [0.26–0.82]; P<0.008).
Intravascular ultrasound (IVUS) and optical coherence tomography (OCT) are two other imaging techniques that have been used to guide and optimise PCI. These modalities provide complementary tomographic imaging of the vessel wall and allow for quantification of atheroma burden and assessment of arterial remodelling. However, by improving anatomic assessment, such modalities have further evidenced the lack of a direct relationship between stenosis severity and myocardial perfusion.45–47 Indeed, hybrid imaging physiological studies have reinforced the concept that stenosis severity does not reliably predict the effects on myocardial blood flow48–51 and that factors other than stenosis severity are better predictors of adverse events.52,53 Indeed, in contrast with the linear model originally proposed by Gould et al.,54 when tested in the clinical settings, the relationship between stenosis severity and the impact on coronary myocardial blood flow is characterised by large scatter.45,46 Actually, while many patients with angina do not display obstructive CAD, stable coronary plaques may also be completely clinically silent.18,42,55 In a large registry study of 15,888 patients referred for coronary angiography between 1996 and 2010, the highest rate of obstructive CAD was distributed amongst patients with typical angina. However, obstructive CAD was also identified in 30–50 % of patients with no angina. Therefore, what is the reason to believe that all symptoms in those with typical angina were provoked by obstructive CAD.56 Similarly, it is conceivable that not all patients with epicardial obstructions will benefit from stenosis removal. Indeed, patients with post-PCI angina suffer myocardial ischaemia that is independent from stenosis removal. Despite evidence of an obstructive CAD, alternative mechanisms were responsible for myocardial ischaemia in this patient subset. While obstructive CAD did not prevent their occurrence, its removal enhanced their discovery.
Predicting Post-percutaneous Coronary Intervention Myocardial Ischaemia
As mentioned, patients with persistent angina constitute a significant, although variable, patient population subset. Their recognition is further acknowledged by the conduction of studies that have aimed to determine the underlying mechanisms. However, at the current state of knowledge, we are not able to predict which patients will or will not benefit from revascularisation, thereby constituting the next big challenge.
According to the new paradigm for stable IHD, myocardial ischaemia should be considered a multifactorial disease. These factors appear neither necessary nor sufficient to induce myocardial ischaemia. This is the reason why unifactorial disease-centred care (i.e. search for and removal of obstructive CAD) has been shown to be ineffective in some patients with suspected IHD. Indeed, PCI in stable angina is a ’one-size-fits-all’ approach, useful for patients for whom epicardial stenosis is the cause of symptoms but not helpful for those in whom it is an innocent bystander. Major international guidelines state that revascularisation should be performed in patients with persistent symptoms despite guideline-directed medical therapy (GDMT).57
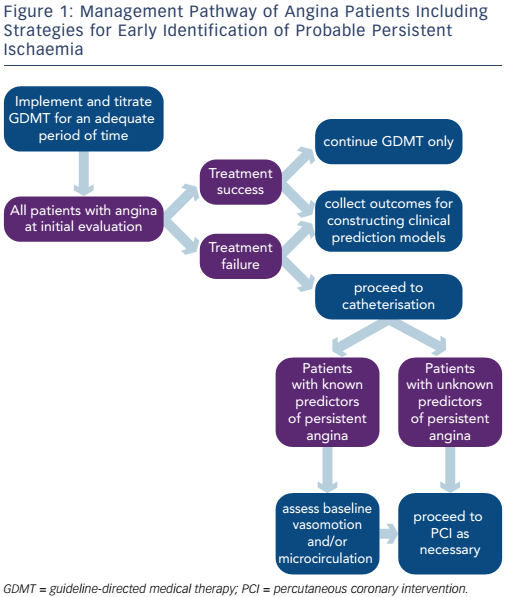
However, revascularisation is often pursued without attempting implementation and/or titration of adequate medical therapy. In a CathPCI Registry® study including 467,211 patients with stable CAD undergoing PCI, less than half were receiving GDMT before PCI and approximately two-thirds were receiving GDMT at discharge following PCI.58 Factors favouring an initial revascularisation strategy include referral bias, financial gain, poor understanding of pathophysiological mechanisms, individual physician belief of what might benefit the patient, and patient perception of the potential benefit of the procedure.59 PCI in stable angina does not prevent death or MI, does not make the asymptomatic patient feel better and has rare but potentially dangerous complications.6 However, most patients undergoing PCI believe that it will reduce the risk of MI and death, 60 and cardiologists continue to perform PCI in patients with minimal or no angina.61 Overrated use with improper selection of patients that may benefit from revascularisation may have contributed to the lack of full-scale beneficial effects of angioplasty. We believe that a more comprehensive utilisation of medical therapy would help understand and predict the effects that a treatment strategy incurs.
In addition, population based studies could be used to construct clinical prediction models for persistent angina. For instance, baseline angina frequency has been shown to be a strong predictor of recurrent angina despite medical treatment or revascularisation.9 Smoking status,62 younger age, and more progressive and severe symptoms prior to revascularisation are other determinants of persistent angina.19
Finally, early identification of patients who are more likely to have persistent angina could be attempted at the time of coronary angiography (i.e. assessment of baseline coronary vasomotor response to ACH or baseline coronary microvascular resistance) (see Figure 1). In a prospective study of 55 patients undergoing PCI for stable angina, resting status of the coronary microcirculation was the strongest independent predictor of post-PCI microvascular resistance.63
Conclusion
Although revascularisation can improve ischaemic symptoms, its effects are not unconditional and currently predictable. These findings are in line with the multidimensional model for IHD, with obstructive CAD constituting only one among other determinants. Therefore, evidence of an obstructive coronary plaque should not be automatically considered as the cause of myocardial ischaemia, but rather considered with appropriate scepticism. The ultimate scope of this approach should not be regarded as an antagonistic view to PCI. On the contrary, a more comprehensive evaluation of patients with IHD would permit a better selection of those in whom a revascularisation strategy is the definite solution, this way better evidencing its benefits.