Non-valvular AF (NVAF) is the most common serious cardiac arrhythmia in developed countries.1 NVAF is associated with an increased incidence of stroke, heart failure and vascular dementia.2 With ischaemic stroke being the main complication, stroke prevention with oral anticoagulant therapy (OAT) has long been the cornerstone of NVAF management. Although NVAF increases the risk of stroke by about fivefold, this risk depends on the concomitant presence of some risk factors.3 The European Society of Cardiology (ESC), the American Heart Association (AHA) and the American College of Cardiology (ACC) all recommend assessing thromboembolic risk on the basis of a patient’s CHA2DS2-VASc score.1,4
NVAF guidelines recommend OAT in men with a CHA2DS2-VASc score of at least 2 and women with a CHA2DS2-VASc score of at least 3. OAT should be considered in men with a CHA2DS2-VASc score of 1 and women with a CHA2DS2-VASc score of 2; OAT is not indicated in other cases.1
For many years, vitamin K antagonists (VKAs) were the only available therapy. When compared with placebo, VKA therapy (mostly warfarin) reduces stroke risk by 64% and mortality by 26%.5 Despite its effectiveness in reducing the risk of stroke, VKA therapy is not free from serious haemorrhagic complications.4,6 Among these, intracranial haemorrhage (ICH) represents the most serious complication.
Non-vitamin K antagonist oral anticoagulants (NOACs) have shown efficacy similar to VKAs, but with a low risk of intracranial haemorrhage (ICH).7–10 Despite these reassuring data, a residual bleeding risk is always a concern.11
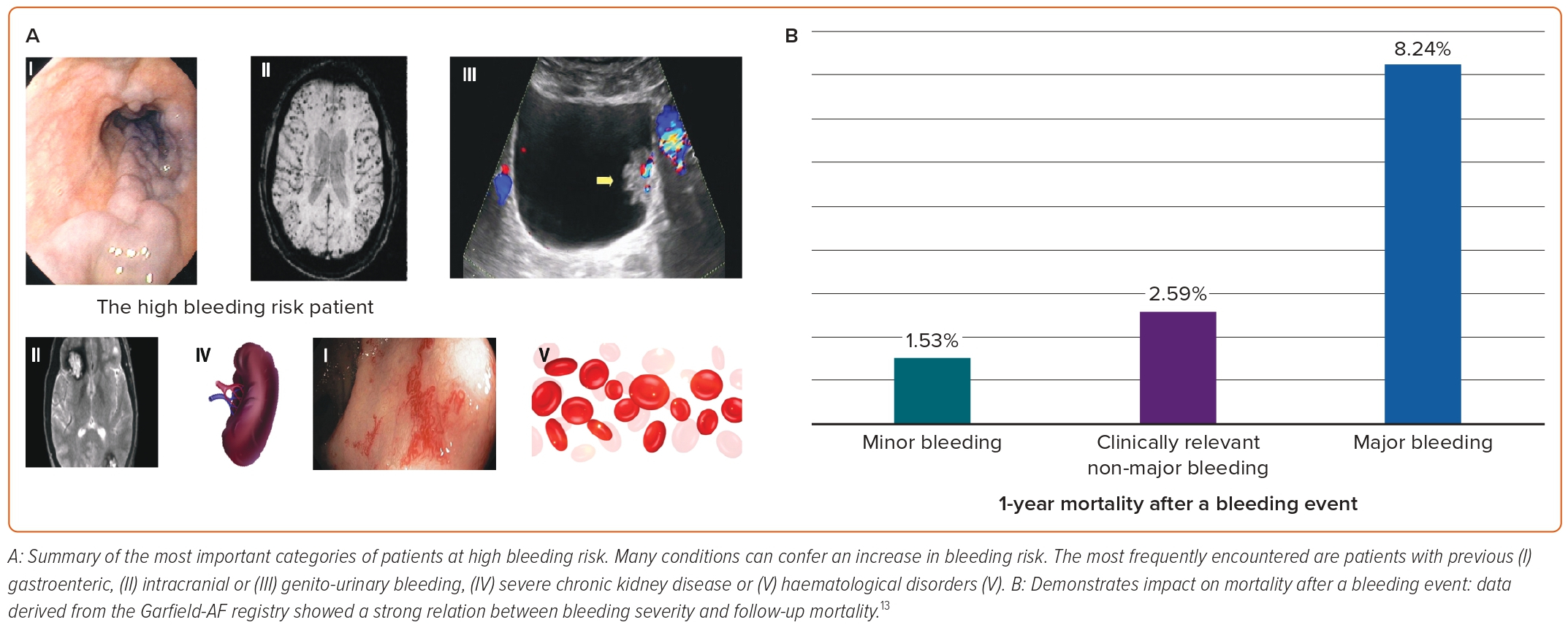
In the real-life Garfield-AF registry, 1-year follow-up event rates of minor, clinically relevant non-major and major bleedings were 2.29, 1.10 and 1.31 per 100 patient-years, respectively.12 The same registry showed a significant impact on mortality after a bleeding event (Figure 1), with 45% of all deaths occurring within 30 days.12 Consequently, the decision to prescribe antithrombotic drugs must necessarily involve an assessment of the risk of stroke against the risk of bleeding.
Which Patients Have a High Risk of Bleeding?
Identifying patients with a high risk of bleeding represents a challenge to the clinician. Guidelines recommend the routine use of the HAS-BLED risk score when evaluating a patient before prescribing OAT for stroke prevention.1,4,14 Although HAS-BLED has the same moderate ability to predict the risk of bleeding as other bleeding scores, it has proven to be the most effective in predicting the risk of ICH. It is also the simplest to use.15 A high score is not a contraindication to OAT, but it can identify patients who deserve greater attention and closer follow-up.1
Beyond bleeding risk scores, some clinical conditions confer an increased bleeding risk per se (Figure 1). Identifying these may represent a contraindication to therapy, irrespective of the score.16
Absolute Contraindications to Oral Anticoagulant Therapy
According to ESC guidelines, absolute contraindications to OAT include active serious bleeding, associated comorbidities, such as severe thrombocytopenia <50 platelets/μl, severe anaemia that is under investigation or a recent high-risk bleeding event such as intracranial bleeding.1
Spontaneous Intracerebral Haemorrhage
The main causes of spontaneous ICH are hypertension, cerebral amyloid angiopathy and anticoagulation.17 Hypertension is the only truly correctable cause and once patients with uncontrolled hypertension have been excluded, the choice to start or resume OAT is difficult.11 Indeed, patients with a history of spontaneous ICH are at high risk of a recurrent event and mortality is high when there is a recurrence.18,19
Some trials that investigated the issue had negative or neutral results. The SoSTART trial showed that 8% of the patients assigned to OAT had recurrence of ICH compared with 4% of the patients who did not start OAT.20 To date, if it is reasonable to restart OAT in patients who experienced deep ICH during severe hypertension, it is not for patients with previous lobar ICH and/or cerebral amyloid angiopathy.21,22 Guidelines recommend that the therapeutic decision should be made by a multidisciplinary team.21,23
Gastrointestinal Bleeding
Nearly half of OAT-associated major bleeding arises from the gastrointestinal (GI) tract, with a higher rate among patients treated with NOACs.24 GI bleedings cause considerable morbidity and mortality (5–15%).25 Despite guideline recommendations, in real life 25–50% of patients do not resume OAT after a GI bleed.25 This is because the source of the bleeding is not identified in 50% of patients and a previous GI bleeding is itself a major predictor of recurrence when restarting OAT.26 Patients with known chronic GI diseases, such as advanced cirrhosis with oesophageal varices, angiodysplasias or active GI cancer, are at higher risk of major GI bleeding with OAT.
Liver Disease
Due to an increase in bleeding risk, NOACs are contraindicated in patients with liver-associated coagulopathy, severe thrombocytopenia and advanced cirrhosis.13 Nevertheless, these patients may be at higher thrombotic risk.27
Chronic Kidney Disease
Patients with severe chronic kidney disease (CKD) have an increased morbidity and mortality due to their excessive risk for thromboembolic and severe bleeding events. Moreover, all NOACs are eliminated by the kidneys and there is no concrete evidence concerning the efficacy/safety ratio of anticoagulants in patients with severe CKD.28
Acquired or Inherited Coagulation Disorders
Patients affected by haemophilia, von Willebrand disease or other major coagulopathy have a particularly high bleeding risk. In these patients, OAT is generally not tested and the optimal therapy should be evaluated by a multidisciplinary team.29
Other Contraindications
There are many non-clinical conditions that can affect bleeding risk.30 Older patients have a higher risk of bleeding and it is necessary to assess frailty and prognosis in this population. Older patients also have an increased fall risk and this could affect the possibility that an anticoagulant drug will be prescribed.31 Some psychiatric patients may have an increased bleeding risk as a consequence of poor adherence to therapy or self-harm.
Resistant Stroke
A completely different indication for left atrial appendage occlusion (LAAO) is represented by those patients who develop a cardioembolic stroke despite adequate anticoagulant therapy.
A significant proportion of patients experience ischaemic stroke despite OAT. In statistical subgroup analyses of primary prevention in randomised trials comparing NOAC versus VKA in patients with AF, 0.7–1.3% of patients experienced ischaemic stroke within the year, whereas in the secondary prevention cohorts, the proportion of patients developing at least one further ischaemic stroke/transient ischaemic attack ranged from 1.8–2.3% per year.32,33
The epidemiological data are reinforced by data deriving from clinical research where it is well known that thrombosis of the LAA can form or persist despite appropriate therapy.34 Moreover, it is now known because the LAA morphology predicts residual stroke risk in AF.34 Reassuring initial data show that the residual risk of stroke can be reduced by LAAO.35
Left Atrial Appendage Occlusion
Left atrial appendage occlusion (LAAO) offers an alternative mechanical approach to reduce cardioembolic risk in AF patients.36
LAA: Embryology, Anatomy and Physiology
The left atrial appendage (LAA) is a complex structure derived from the primitive atrium. During the fourth week of gestation, the primitive atrium performs a right-handed looping in its actual and definitive position and the subsequent cellular replication contributes to form the pectinate muscles, which lend a rough endocardial surface to the LAA.37 The rest of the left atrium is smooth and derived from primordial pulmonary veins (PVs).
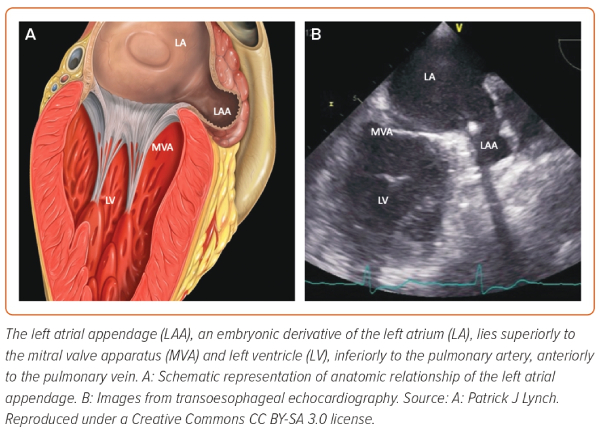
The LAA lies superior to the mitral valve and the atrioventricular groove, inferior to the pulmonary artery and anterior to the PVs. On the epicardial side, the ligament of Marshall separates the LAA from the PVs forming the coumadin ridge (Figure 2).
The LAA is highly variable in size and morphology. The shape of LAA ostium is elliptical in 68.9% of cases, while a round shape is observed in 5.7%. The length of the LAA is on average 45 mm long, ranging from 27–60 mm.37 In a large series of 500 autopsies, the LAA was bi-lobed in 54%, tri-lobed in 23% and single-lobed in 20% of cases.38
From a physiological perspective, the LAA has both mechanical and endocrine properties. It acts as a reservoir during ventricular systole, a conduit for blood passage from the pulmonary veins to the left ventricle during early diastolic phase and it is an active contractile structure in atrial systole.39 The LAA is more distensible than the rest of the LA and may represent a volume reserve.40 The endocrine function of the LAA is related to the secretion of natriuretic peptides as a response to stimulation of stretch-sensitive receptors. Indeed, the LAA contains about 30% of all cardiac atrial natriuretic peptides and the increase in LAA pressure results in diuresis, natriuresis and increased heart rate.41,42
Relationship between the LAA and Stroke: a Rationale for LAA Occlusion
The LAA is the source of 90% of the thrombi in NVAF.43 The explanation is summarised by Virchow’s triad: stasis of blood, hypercoagulability and endothelial injury.44
Thrombosis within the LAA happens more frequently when reduced contractility and stasis occurs. During NVAF, the reduction of LAA peak flow velocity is one of the strongest predictors of an increased thromboembolic risk.45 LAA geometry and haemodynamics can be viewed independently of CHA2DS2-VASc score, with the combination of LAA orifice >4 cm2 and LAA flow velocity <40 cm/s being significantly associated with stroke risk with added risk for patients with added risk for patients with a CHA2DS2-VASc score 0–1.46 Endothelial injury of the LAA during NVAF has been demonstrated in autopsy and in biopsy findings.47 A hypercoagulable state in NVAF has been shown by an increase in blood markers indicating coagulation activity.36
LAA Morphology and Thromboembolic Risk
Modern imaging techniques have allowed us to classify the LAA in four main morphologies:36
- The chicken wing shape (48% of cases) has a central lobe and a bend in the middle with the possibility of secondary lobes;
- The cactus morphology (30% of cases) has a central lobe with multiple secondary lobes that have different spatial orientations;
- The windsock shape (19% of cases) has a dominant central lobe which may have some secondary lobes;
- The cauliflower shape (3% of cases) does not have a dominant lobe, and has multiple lobes.
The morphological variants of the LAA appear to influence the risk of stroke. Assuming chicken wing variant as reference, the cactus morphology was associated with a 4.08-fold greater risk, the windsock with a 4.5-fold greater risk and the cauliflower with an eightfold greater risk of stroke or transient ischaemic attack. In a sub-analysis including only patients with a CHADS2 score 0–1, the presence of a non-chicken wing morphology was an independent predictor of stroke (OR 10.1; 95% CI [1.25–79.7]; p=0.019).48
Devices for LAA Occlusion
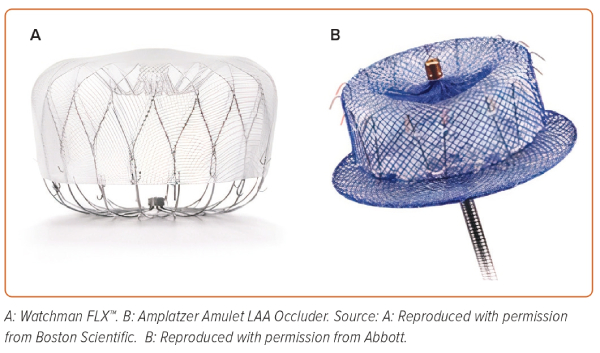
The LAA occlusion devices (Figure 3) are based on three different principles:
- Plug: lobe or umbrella occluding the neck of the LAA (WATCHMAN – Boston Scientific, WaveCrest – Biosense Webster).
- Pacifier principle: the device is formed by a lobe or umbrella with an additional disc that seals the ostium of the LAA from the left atrial side (the Amplatzer Cardiac Plug and Amulet – Abbott Vascular; Ultrasept LAA Occuluder – Cardia; or LAmbre – Lifetech).
- Ligation: the device snares and ligates the neck of the LAA using a combined endocardial and epicardial approach (Lariat – SentreHEART).
The endovascular devices – the plug and pacifier devices – allow a technical feasibility in more than 95% of patients and contraindications are rare. Epicardial ligation is not commonly used and 25% of patients are not eligible due to anatomical contraindications.36
LAA Occlusion Planning
Once the aspects relating to the patient are well understood, diagnostic imaging is essential for accurate procedural planning.
Key elements of the pre-procedural planning involve:
- Ruling out the presence of LAA thrombosis: this is generally a major contraindication to the procedure;
- Knowing the real anatomy of the LAA and interatrial septum;
- Defining the measurements of the ‘landing zone’ and the ostium.49
This information will allow the interventional cardiologist to choose the safest approach and the device that best fits the patient’s anatomy.
For these purposes, transoesophageal echocardiography is generally the gold standard and the most used method.49 An alternative option is cardiac CT, which allows a quick and non-invasive assessment of LAA anatomy. The development of dedicated software and protocols will probably lead to the emergence of cardiac CT as the method of choice.50
Standard Technique
Percutaneous LAA occlusion is generally performed under transoesophageal echocardiography (TEE) guidance with general anaesthesia. The right femoral vein is the vascular access of choice. After advancing a J-tipped guidewire from the right femoral vein into the superior vena cava, the transseptal sheath and dilator were advanced over this wire. After the guidewire is removed, a needle connected to a pressure line is inserted. The transseptal puncture should ideally be performed in the postero-inferior area of the fossa ovalis to obtain an optimal coaxiality with the LAA, which is oriented anteriorly and superiorly. The achievement of an inferior location is evaluated under TEE guidance in bicaval view. After transseptal puncture, heparin is administered to achieve an activated clotting time >250 seconds and transseptal sheath (TSS) is advanced in the LA. After advancement of the TSS in the LA, a stiffer wire is advanced in the left superior pulmonary vein to exchange the TSS with the delivery sheath. After sizing with TEE and angiography, the LAA occlusion is performed according to the implantation technique of the chosen endovascular device. After deployment of the LAA occluder in a satisfactory position, the stability of the occluder should be checked before proceeding to the final release.
Possible Evolution of the Technique
The traditional approach to LAAO is based on TEE guidance and typically requires an anaesthetic and tracheal intubation. This is both a logistical and a clinical problem, as many of the patients are extremely frail. In recent years, the significant improvement in intracardiac echocardiography (ICE) has allowed the development of a minimalist approach to LAAO. Indeed, ICE guidance allowed the procedure to be performed under local anaesthesia and without tracheal intubation.51 As this approach could reduce patient discomfort, ICE could become the new gold standard. Nowadays, this approach is only for selected patients as TEE guidance is generally believed to be superior.
Procedural Complications
The incidence of procedure-related complications in clinical and registry studies is constantly decreasing due to the learning curve. In the large EWOLUTION registry, the most frequent complications observed were major bleeding (0.6%), pericardial effusion (0.4%, including only one cardiac tamponade), vascular damage to the groin (0.4%), procedural air embolism (0.3%) and device embolisation (0.2%).52 The risk of procedural ischaemic stroke was <0.5%.52
Antithrombotic Therapy after Percutaneous LAAO
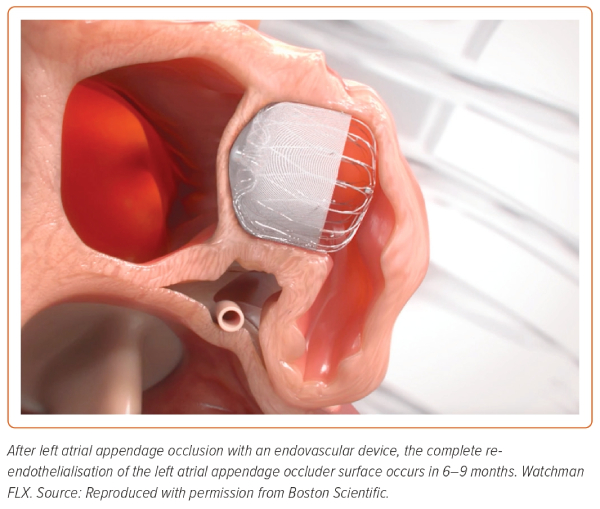
After LAAO, antithrombotic therapy is recommended to prevent thrombus formation on the surface of the device until its complete endothelialisation (Figure 4).16 The optimal antithrombotic strategy after LAA is unclear. The current management protocols are based on trials of the Watchman device and on experience gained by using other cardiac devices, such as a patent foramen ovale occluder and bioprosthesis valve.
Warfarin should be given for 45 days (international normalised ratio target 2–3) after Watchman implantation in patients suitable for OAT, followed by clopidogrel for at least 6 months. Direct oral anticoagulants (DOACs) could be used as an alternative to warfarin.
In patients not suitable for OAT, such as those with a high risk of bleeding, a short course (1–6 months) of dual antiplatelet therapy with acetylsalicylic acid and clopidogrel is generally recommended after Watchman implantation.36 Due to registration design studies, a short course (1–6 months) of dual antiplatelet therapy with acetylsalicylic acid and clopidogrel is generally recommended for all patients after Amulet or ACP implantation.
Despite these recommendations, there are no randomised data that allow us to conclude with certainty which is the optimal therapy.53 Despite the absence of data deriving from randomised controlled trials, there is a substantial amount of evidence from prospective studies supporting the safety of using antiplatelet therapy rather than anticoagulant therapy after the procedure.52,54
In selected cases of very high bleeding risk, a single antiplatelet therapy could be prescribed.36 There are anecdotal reports where no antithrombotic therapy has been prescribed for patients with active bleeding.55
Summary of LAAO Evidence in Patients with a High Risk of Bleeding
The most important clinical studies supporting LAAO efficacy and safety are the PROTECT AF and the PREVAIL studies in which patients who were eligible to use VKAs were randomised to either the Watchman device or VKAs.56 Despite a debated statistical methodology, these are the only randomised trials evaluating LAAO versus VKA therapy.
Globally, LAAO proved to be non-inferior to VKAs in preventing ischaemic stroke or peripheral embolism, with a significant reduction in haemorrhagic complications. However, although the difference is not significant, the systemic embolism rate was numerically higher with LAAO. As a consequence of statistical methodology, a small number of patients enrolling and an absence of a comparison with NOACs, these results could not be generalised and OAT remains the optimal therapy for NVAF patients with an increased embolic risk.36
PRAGUE-17 was a randomised, non-inferiority comparison of percutaneous LAAO versus NOACs.57 In this trial, after a follow-up of about 4 years, LAAO remained non-inferior to NOACs for the composite endpoint of stroke, transient ischaemic attack, systemic embolism, cardiovascular death, major or non-major clinically relevant bleeding, and procedure-/device-related complications. Despite these interesting results, the PRAGUE-17 study was too small to draw definitive conclusions.58
To date, there are no randomised data to compare the efficacy of LAAO in patients who cannot tolerate even short-term anticoagulation. In the absence of randomised trials, many registries had reported outcomes in patients deemed ineligible for OAT.
The EWOLUTION trial was a prospective multicentre registry study that enrolled patients at very high risk for thromboembolism (mean CHA2DS2-VASc score 4.5) and major bleedings (15.1% had experienced a previous haemorrhagic stroke and 31.3% had a history of major bleeding). The results showed that LAAO with Watchman is safe and effective, with an ischaemic stroke rate that was as low as 1.3% and major bleeding reduction of about 50%.59 Another large prospective European registry reported similar results in 1,088 patients undergoing LAA occlusion with the Amulet device.54
Other small registries had evaluated LAAO outcomes in selected populations. Of relevance are results in patients where OAT is absolutely contraindicated for previous ICH and has a higher risk of recurrence, such as patients with cerebral amyloid angiopathy, LAAO has been shown to be an effective option in the prevention of thromboembolic complications for this frail population.60,61
Another population frequently encountered in clinical practice is NVAF patients with concomitant end-stage renal disease, in whom OAT is ineffective or contraindicated. A prospective multicentre study suggested that LAA occlusion is feasible and safe in patients undergoing dialysis and that it is associated with a reduction of thromboembolism compared to non-treated patients, and of haemorrhagic events compared to patients taking OAT.62
Reassuring data are derived also by patients in which OAT was contraindicated by frequent GI bleeding. In this population, LAAO is associated with a low annual rate of stroke/transient ischaemic attack, with a 20.1% relative risk reduction of annual major bleeding rate.63
Current Guidance
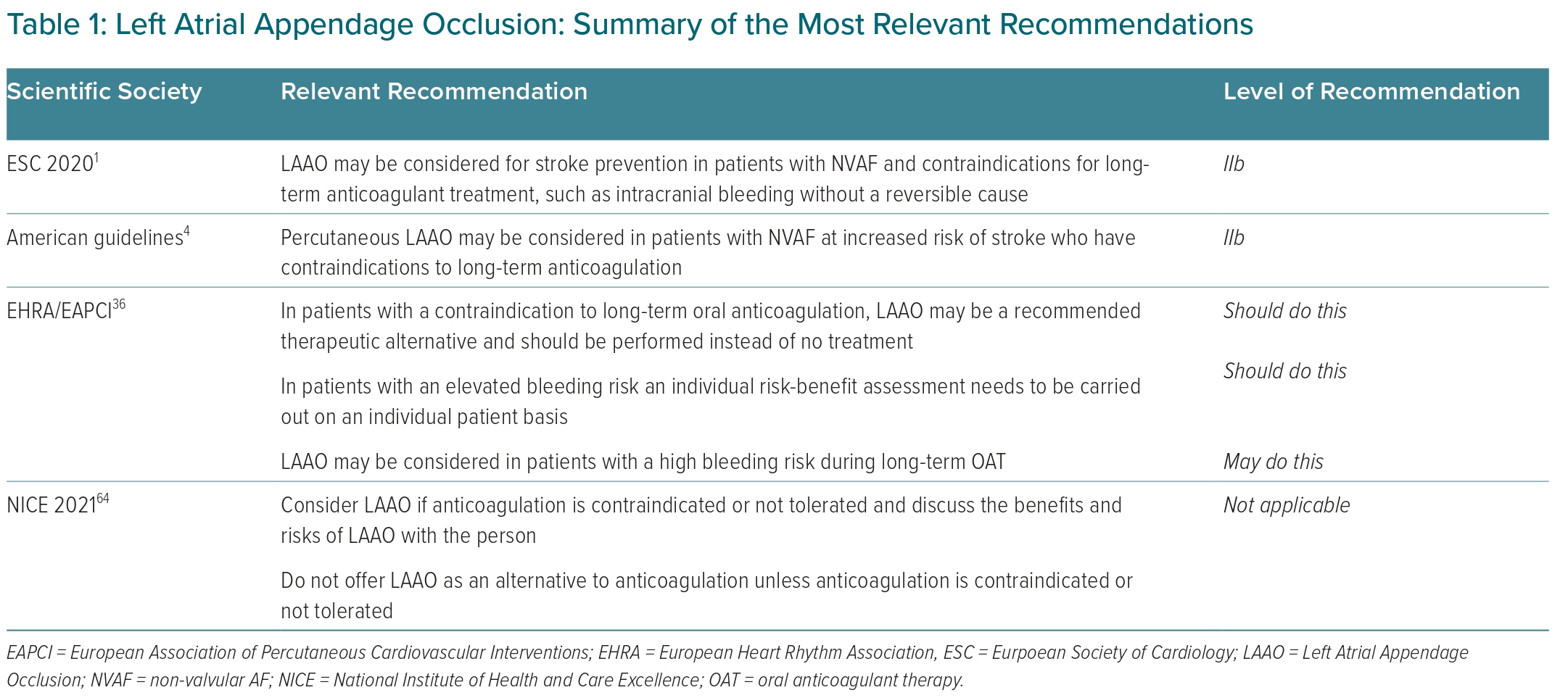
Indications for percutaneous LAAO are various and sometimes debated. Guidelines and consensus documents of most scientific societies have tried to identify patients who could benefit from the procedure (Table 1).1,4,36,64
According to the latest ESC guidelines for the management of AF, percutaneous LAAO may be considered for stroke prevention in patients with NVAF and contraindications for long-term anticoagulant treatment (evidence class IIb, level B).1 This restrictive indication is due to a lack of randomised trials.
Similarly, 2019 American guidelines established that percutaneous LAAO may be considered in patients with AF (NVAF) at increased risk of stroke who have contraindications to long-term anticoagulation (class of recommendation IIb; level of evidence B).4 The document underlines that the Watchman device provides an alternative to OAT for stroke prevention in most patients with NVAF and elevated stroke risk but who are poor candidates for long-term OAT due to bleeding risk or low therapy adherence/tolerance.
In 2020, the European Heart Rhythm Association and the European Association of Percutaneous Cardiovascular Interventions issued a consensus statement that identified the characteristics of patients undergoing LAAO.36 In particular, the document focuses on some patient categories, such as those with NVAF who are eligible for chronic long-term OAT, patients with a contraindication for OAT, patients with an elevated bleeding risk under chronic OAT, patients who are unwilling or unable to take OAT, and specific subgroups, such as OAT not efficient or ‘stroke despite taking OAT’, electrically isolated LAA post-catheter ablation, the combination of AF ablation and LAA occluder implantation and LAA closure for primary prevention.36
Guidelines from the National Institute for Health and Care Excellence recommend considering LAAO in patients with contraindication or intolerance to anticoagulation therapy after appropriate discussion of the procedure with the patient. This document recommends to not routinely offer LAAO as an alternative to OAT. Of interest, NICE guidance recommends that LAAO should be offered to patients with an adequate quality of life and with a life expectancy of at least 3 years.64
Despite the weak level of recommendation, the demand for LAAO procedures from clinicians is growing exponentially.
Future Directions
Despite the absence of randomised trials and weak guideline recommendations, the number of LAAO procedures is growing worldwide. This is the consequence of the response to the clinical need to reduce the embolic risk in those patients in whom OAT is contraindicated.
The advent of a less invasive approach, essentially based on the use of intracardiac ultrasound, could lead to a further expansion of the population that could benefit from the procedure.
A major issue remains over the optimal post-LAAO therapy and how to address the residual embolic risk. If it is true that the LAAO excludes the main embolic sources during AF, it is equally true that the procedure is unable to modify the residual risk deriving from a concomitant atrial cardiomyopathy or from a systemic prothrombotic state.
Of interest, some authors have recently demonstrated that after the procedure, long-term half-dose DOAC significantly reduced the risk of embolism and major bleeding events compared with an antiplatelet-based antithrombotic therapy.65
Another topic of future research is represented by patients with resistant stroke. Combining LAAO and oral anticoagulation could potentially give the best results in terms of secondary prevention of embolic stroke. It would be interesting if a similar approach could be tested in a randomised primary prevention trial.
Conclusion
NVAF is a common clinical condition and its prevalence is growing as a consequence of an ageing population. Although OAT is the cornerstone of NVAF management, many patients cannot receive this therapy because of bleeding or a high risk of bleeding.
LAAO is a nonpharmacological option for preventing cardioembolic events in patients with NVAF at significant stroke risk and with a contraindication to OAT, and clinicians should consider this alternative option when managing these patients.