Atherosclerosis is an important health and economic burden worldwide. Unfortunately cardiovascular scores based on usual risk factors lack specificity and so does the identification of unstable carotid plaques. Currently it is still a major issue to identify high cardiovascular disease risk individuals and high-risk carotid plaques. Ultrasounds can provide qualitative and quantitative data on carotid arteries and plaques. Other imaging techniques (for example, computed tomography [CT] scan, magnetic resonance imaging [MRI] and positron emission tomography [PET] scan) can be useful for evaluating the severity of carotid stenosis and the stability of carotid plaques, but also for detecting subclinical atherosclerosis and improving the classification of individuals at risk of developing cardiovascular disease.
Identification of the Unstable Carotid Plaque with Ultrasounds
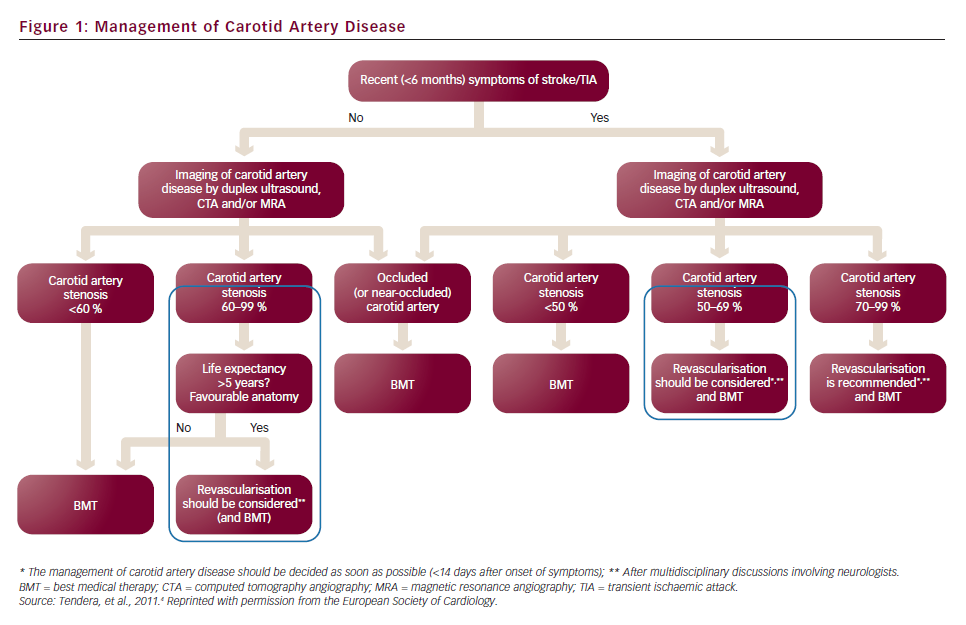
In patients with symptomatic carotid stenosis >50–70 %, endarterectomy is generally accepted as being cost-effective. However, the cost-effectiveness of surgery is less clear for asymptomatic patients1 whose ipsilateral stroke risk is 2 % per year2,3 or even less with current effective medical therapies (best medical treatment [BMT]). Nevertheless, though the number needed to treat (NNT) by surgery to prevent disabling stroke or death in one patient over a five-year period is high (NNT=32), only 15 % of strokes are preceded by transient ischaemic attack (TIA). The latest algorithm of the European Society of Cardiology (ESC) for the management of extracranial carotid artery disease (2011) is illustrated in Figure 1.
The severity of carotid stenosis is no longer the only parameter to be taken into account. However, are we currently able to detect at-risk patients or inversely, are we able to select low-risk patients (with a stroke risk <1 % per year) and spare them from an unnecessary endarterectomy? That is one of the current major issues of carotid disease imaging.
Histology taught us that unstable plaques usually have an important lipid core, a thin fibrous cap, inflammatory cells and enhanced metabolism, neo-angiogenesis and sometimes endothelial erosion, intraplaque haemorrhage or thrombosis. Attempting to identify unstable plaques in vivo stimulated imaging research (CT scan, PET scan, magnetic resonance angiography [MRA] and ultrasounds). Hereafter I will focus on ultrasounds.
Routine Ultrasound Imaging Techniques
Several complementary sonographic techniques are now available for the evaluation of the extracranial arteries.
Brightness Mode and Pulsed Wave
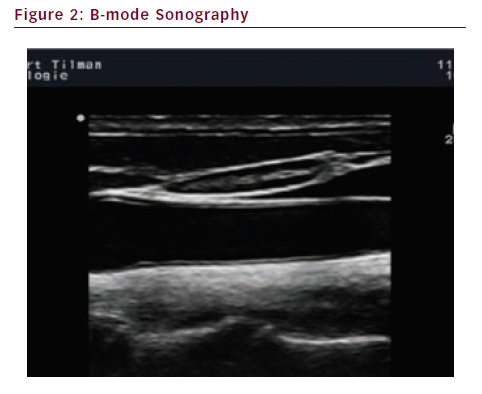
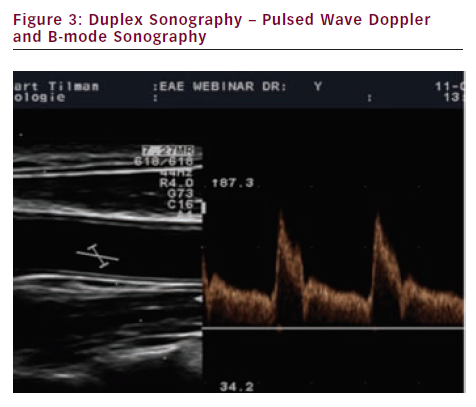
High-resolution brightness mode (B-mode) scanning is used to display the morphological features of vessels (see Figure 2). Duplex sonography combines pulsed wave (PW) Doppler spectrum and B-Mode sonography (see Figure 3).
Colour Doppler Flow Imaging
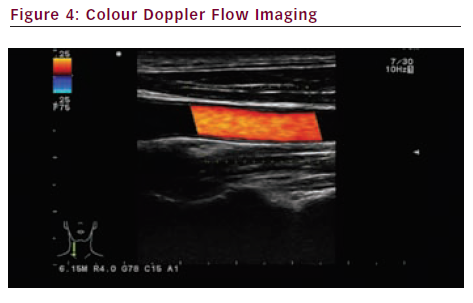
Colour Doppler flow imaging (see Figure 4) adds the advantages of conventional duplex sonography to colour-coded blood flow patterns. The major advantages are: the colour code gives information on the direction of blood flow; it can measure the average mean velocity of moving blood cells within the sample volume at a given point in time; and aliasing helps detecting stenoses (high velocity). The drawbacks include weak spatial and temporal resolution.
Power Doppler
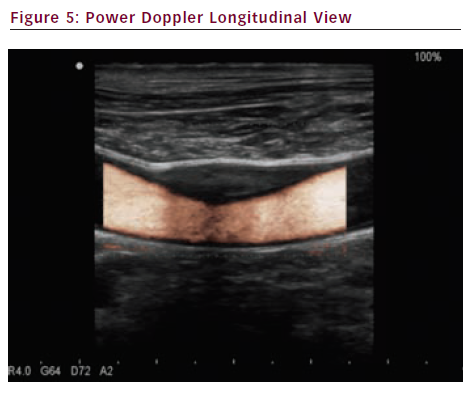
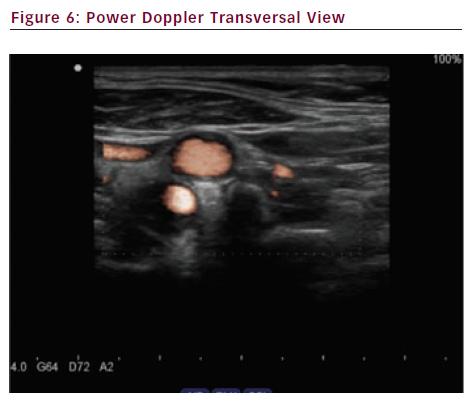
Power Doppler (see Figures 5 and 6) also called angio Doppler because it looks like an angiography, has many advantages such as no aliasing, detection of low velocity flows and least angle-dependence allowing a better display of curving or tortuous vessels and a better assessment of plaque surface structure. Drawbacks include no information on flow direction.
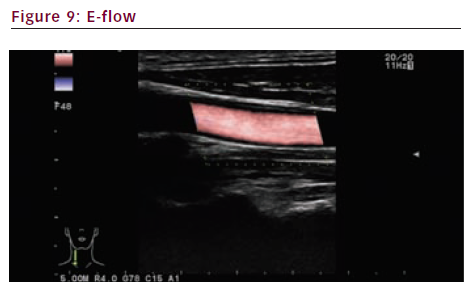
B-flow and E-flow
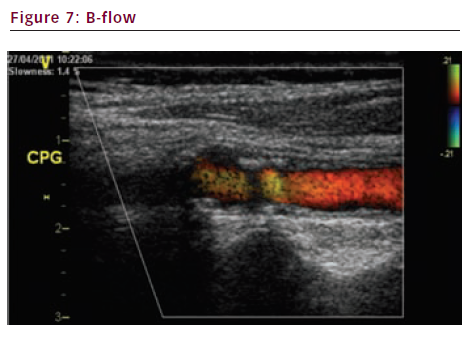
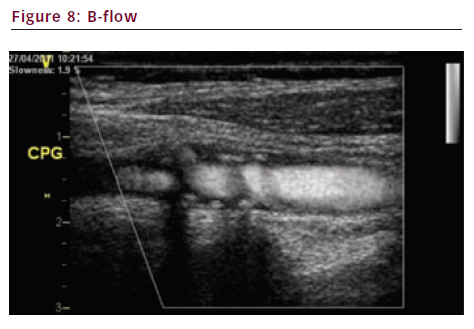
Brightness flow (B-flow) (see Figures 7 and 8) and extended flow (E-flow) (see Figure 9) are new imaging techniques. They give a very precise display of the vessel wall and the surface of the plaque.
Characterisation of the Plaque
Some echographic features reflect a higher vulnerability of the plaque.
Echogenicity
Homogeneous (iso- or hyperechogenic) plaques correspond to fibrous tissue and are less harmful than echolucent ones, which correspond to lipid deposits, necrotic residuals or intraplaque haemorrhage. Plaques can be classified according to their echogenicity (modified version of Gray-Weale’s classification5,6):
- type 1 plaque: uniformly echolucent;
- type 2 plaque: predominantly echolucent;
- type 3 plaque: predominantly echogenic;
- type 4 plaque: uniformly echogenic; and
- type 5 plaque: unclassifiable because of heavy calcification and acoustic shadows.
Types one and two are supposed to be more at-risk than the others.
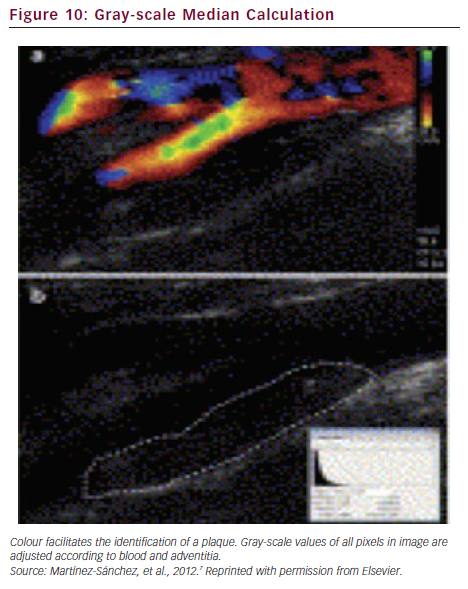
To reduce the subjectivity bias of this classification, plaque echogenicity can also be quantified by computerised analysis with the gray-scale median score (GSM), i.e. the median value of the pixel intensities for the entire plaque (see Figure 10).7 The correlation between low GSM scores and stroke risk has been proved in symptomatic carotid stenosis but the technique is not sufficiently reliable to be translated into clinical practice.
Plaque heterogeneity can be represented by colour mapping, based on computer-assisted analysis of local density (i.e. the distribution of pixel intensities). This representation could better predict the instability of the asymptomatic carotid plaque than GSM.8 The degree of stenosis and predominant low-density areas on plaque surface are independent factors associated with cardiovascular events and/or lesions on cerebral MRI.
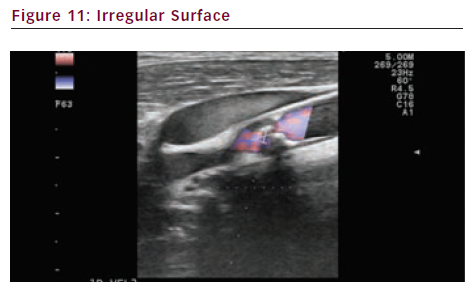
Surface Irregularity and Anfractuosity
An irregular surface with anfractuosity can correspond to an ulcerated unstable plaque. The surface of the plaque can be evaluated with colour Doppler, or more accurately with power Doppler, E-flow (see Figure 11) or B-flow. Contrast enhanced ultrasounds (CEUS) can also optimise the visualisation of luminal irregularities.
Ultrasound Contrast Agents
Ultrasound contrast agents (mostly SonoVue®) are gas-filled microbubbles with a diameter less than that of redblood cells. They are contraindicated in case of severe cardiac failure or coronary disease, in pregnant or breast-feeding women and in children. Clinical applications of carotid CEUS include:
- enhancement of the carotid artery lumen enabling better visualisation of echolucent plaques, irregularities, ulcerations and dissections;
- improved precision of measurement of carotid intima-media thickness (CIMT);
- Doppler rescue in case of unsatisfactory echogenicity (rarely needed in experienced hands);
- better efficiency of transcranial Doppler (TCD); and
- plaque neovascularisation.
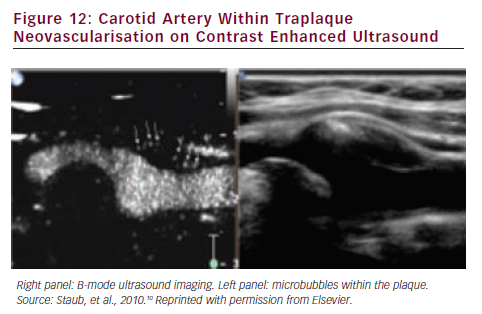
Neovascularisation
Neovascularisation consists in an intraplaque-increased number of immature microvessels that may cause the ‘leak’ of noxious inflammatory plasma components, plaque inflammation and growth and sometimes intraplaque haemorrhage. Consequently the degree of plaque neovascularisation is directly associated with cardiovascular disease (CVD) and cardiovascular events (CVE).9 Intraplaque angiogenesis can be detected by CEUS (see Figure 12).
Unfortunately, the quantification of the neovascularisation is a major issue confronting 2D-CEUS as a clinically useful imaging technique. The advent of 3D and other technical evolutions will probably help detecting and quantifying the neo-angiogenesis, but currently a quantifiable volumetric analysis of neovascularisation remains elusive.10
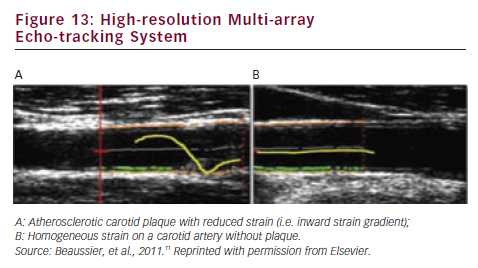
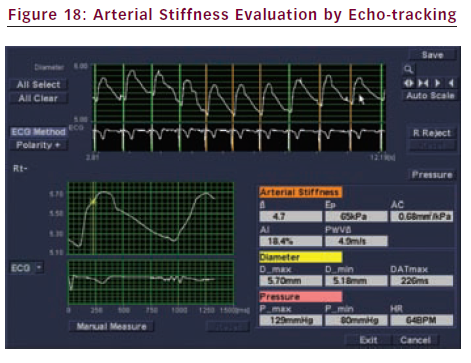
The mechanical parameters (distensibility etc.) of the carotid plaque and the adjacent carotid common artery can be evaluated with high-resolution echo-tracking (see Figure 13). Reduced strain, associated with an outer remodelling, may be a feature of high-risk plaques.11
Transcranial Doppler
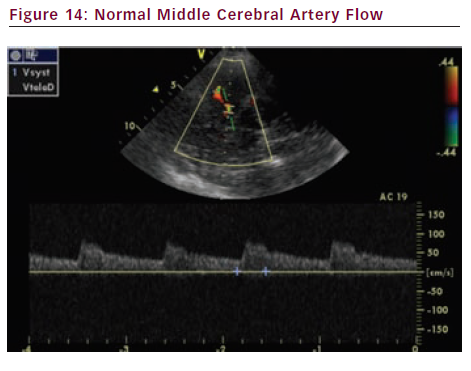
Transcranial Doppler (TCD) measures blood flow velocity and direction in the proximal portions of large intracranial arteries (see Figure 14). TCD can be useful to evaluate several diseases. As far as carotid disease is concerned, let us mention those outlined below.
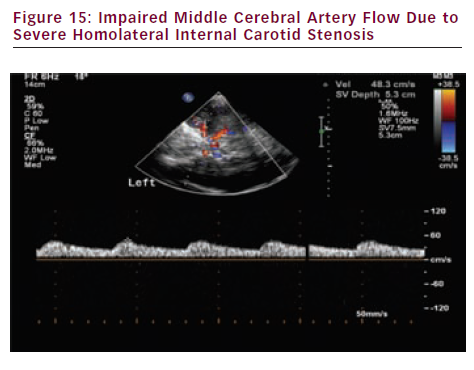
- Evaluation of cerebral haemodynamic impairment distal to severe extracranial internal carotid artery (ICA) stenosis or occlusion (middle cerebral artery flow asymmetry – peak systolic velocity and systolic ascension time) (see Figure 15).
- Assessment of vasomotor reactivity (VMR) with the Breath Holding Test (BHT) or other more sophisticated tests – VMR represents the cerebral autoregulation to maintain cerebral blood flow stability. Reduced VMR is correlated with the degree of ICA stenosis and might predict the risk of future stroke in patients with high-grade ICA stenosis. However, currently there are insufficient data to justify the routine clinical use of VMR to stratify risk in patients with asymptomatic carotid stenosis for selection for carotid endarterectomy.12
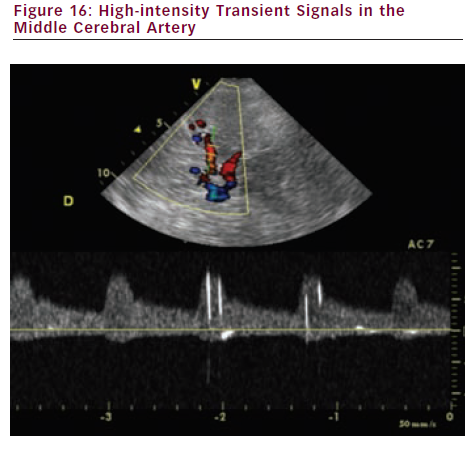
- Assessment of micro-embolic signal (MES) detection. Asymptomatic circulating cerebral emboli can be detected by TCD after one hour of monitoring. MES appear as high-intensity transient signals (HITS) (see Figure 16) and independently predict two-year stroke risk. Detection of MES can be used to identify patients with asymptomatic carotid stenosis who are at a higher risk of stroke and TIA, and also those with a low absolute stroke risk (Asymptomatic Carotid Emboli Study [ACES]).13
Cardiovascular Risk Stratification
Traditional cardiovascular risk factors (CVRF) lack specificity with a significant overlap between CVRF status among individuals that will and will not develop cardiovascular disease (CVD). Carotid arteries can be used as privileged windows of subclinical atherosclerotic disease. Atherosclerosis is an evolving process. In fact the arterial wall disease already starts before wall thickening appears.
Echo-tracking
Local arterial stiffness can be measured in the carotid artery with echo-tracking (e-tracking), a technology using radio frequency (RF) signals. The tracking gate always traces the same position on the vessel wall corresponding to the movement by the beating of the heart (see Figure 17). The stiffness parameter (β) is calculated from changes in vessel diameter and blood pressure (see Figure 18):
β=ln(Ps/Pd)/[(Ds–Dd)/Dd]
When the vessel becomes stiffer, the β is higher. β increases with age. Exceeding the mean value of 10, the artery may be considered to become stiff. CVRF such as smoking, hyperlipidemia, menopause and others are also reported to increase β.14
Augmentation Index
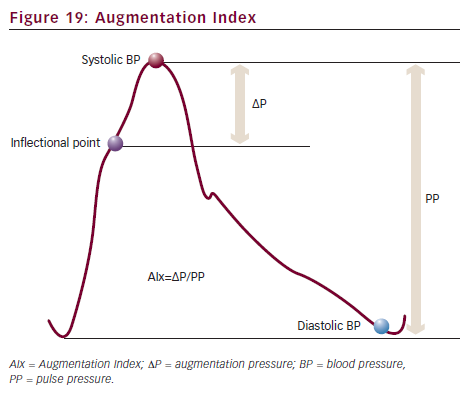
Any blood pressure curve consists of two components. One is a forward wave coming from the heart in early systole. The other is a reflected wave returning from the peripheral arteries towards the heart in late systole. On the blood pressure curve, there is an inflectional point that shows the beginning point of the reflected wave (see Figure 19). Augmentation Index (AIx) represents the ratio of reflected wave. It can be an index of arteriosclerosis.
Speckle Tracking
This technique tracks specific acoustic markers (speckles) in the grey-scale image, frame-by-frame, throughout the cardiac cycle (see Figure 20).15 Speckle tracking provides angle-independent calculations of velocity, displacement, circumferential strain and strain rate. It is a valuable tool for the assessment of the arterial stiffness and the assessment of intraplaque deformation parameters (and maybe prediction of plaque rupture?).
Carotid Intima-media Thickness and Carotid Plaques
During the last decades, CIMT and presence of carotid plaques did emerge as widely accepted surrogate markers of atherosclerosis, a carotid plaque being generally more predictive of coronary heart disease (CHD) risk than CIMT.
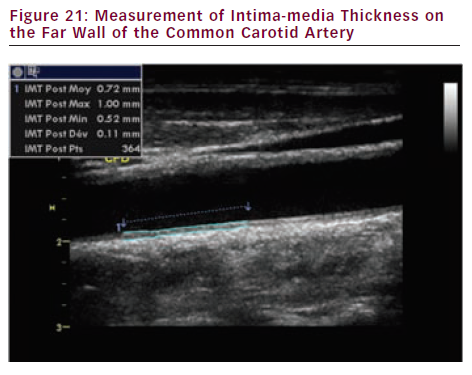
CIMT is defined as the distance between the lumen-intima interface and the media-adventitia interface (see Figure 21). Today CIMT measurement is most commonly performed semi-automatically on the far wall of the common carotid artery (minimum 10 mm length sample) with a 7.5–10.0 MHz linear probe on a longitudinal view.
Carotid plaque is commonly defined as a focal structure that encroaches into the arterial lumen of at least 50 % of the surrounding CIMT value or demonstrates a thickness >1.5 mm (Mannheim consensus).16,17 Since 2000 several guidelines or consensus statements have recommended CIMT measurement and/or carotid plaque detection as clinical tools to improve CVD risk assessment. However, the US Preventive Services Task Force (USPSTF) recently recommended against measurement of anatomic markers of atherosclerosis, including CIMT. The USPSTF’s negative recommendation was based on:
- the absence of specific data regarding the independent predictive value of CIMT for patients at intermediate CHD risk;
- concerns about this test’s ability to reclassify patients into lower or higher risk categories;
- the inclusion of patients with pre-existing CHD or risk equivalent conditions in some studies associating CIMT with CHD risk;
- the relatively short duration of studies; and
- the small number of CHD events.18
The Atherosclerosis Risk In Communities (ARIC) study19 included 13,145 subjects who were free of prevalent CHD or stroke with a mean follow-up duration of 15 years. The ARIC study results support the American Society of Echocardiography’s (ASE) recommendation of combining CIMT and carotid plaque data for optimal risk prediction. For the carotid ultrasound data presented by Nambi et al. (ARIC), the conclusions are clear; large numbers of persons in the intermediate-risk classifications were reclassified when CIMT and plaque data were considered. Amazingly, most of the reclassified patients moved into a lower risk stratum, rather than a higher risk stratum. 3D ultrasonography (US) can evaluate the regression and the movement of carotid plaques more accurately than 2D-US. 3D-US could become a valuable tool for research and drug trials.20
Conclusion
Discriminating between stable and unstable carotid plaques as well as improving the cardiovascular risk evaluation of intermediate risk populations is a new challenge for imaging techniques (especially for carotid ultrasounds). To convincingly demonstrate their reliability they will require prospective randomised studies comparing a strategy of imaging-guided risk factor modification to risk factor modification alone.