The growing incidence of aortic stenosis (AS) in line with the increasingly elderly population has led to the aortic valve being the most commonly treated valve in Europe and North America, via both surgery and through percutaneous approaches.1 Since the first transcatheter aortic valve implantation (TAVI) procedure in 2002, application of this technique to all risk settings has rapidly advanced.2,3 In parallel, there has been an increase in off-label indications for this technology. Such indications have spearheaded the more frequent recommendation for TAVI in clinical practice guidelines in some scenarios. Nevertheless, in other scenarios, the current technology has not yet reached the point of being a better option than the classical alternative – surgical aortic valve replacement (SAVR).
New indications – as well as alternative approaches for TAVI – have progressively expanded, preceded by application in compassionate-use settings. In this review, we aim to explore in detail the current boundaries of these indications by examining the main off-label uses of TAVI and the reported outcomes in such challenging scenarios.
Special Scenarios for TAVI
Several controversial indications currently exist for the TAVI procedure. In this review, we focus on the following scenarios: TAVI for valve-in-valve (ViV) procedures, TAVI for bicuspid AS and TAVI for pure aortic regurgitation (AR). Finally, recently developed implantation strategies are described in a dedicated section.
TAVI for Valve-in-valve Procedures
Structural Valve Deterioration of Bioprostheses
Compared with mechanical valves, bio-prostheses have limited durability, eventually failing within 5–20 years of the intervention. However, in these circumstances, treatment with ViV procedures can be used, as opposed to mechanical valves. Furthermore, bio-prostheses do not require the use of anticoagulation, minimising the associated risks.4 These factors have led to a significant increase in their use in the last two decades.
Structural valve deterioration (SVD) is an acquired intrinsic bioprosthetic valve abnormality defined as deterioration of the leaflets or supporting structures resulting in thickening, calcification, tearing, or disruption of the prosthetic valve materials with eventual associated valve haemodynamic dysfunction. Mechanical stress, collagen fibre disruption and tissue calcification are the main elements involved in this process. Although there is not a standard definition for SVD, Dvir et al. proposed a practical definition of SVD in the Valve-in-Valve International Data registry and provided recommendations for the timing of clinical and imaging follow-up assessment.5 This definition describes SVD as a continuum instead of a binary categorical variable. Stage 1 correlates with early morphological leaflet changes, without haemodynamic sequelae. Stage 2 refers to morphological abnormalities of valve leaflets associated with haemodynamic dysfunction. This stage is divided according to the type of dysfunction – either stenosis (Stage 2S) or regurgitation (Stage 2R) – because the clinical implications and speed of deterioration are likely to differ between these two failure modes. Investigators categorised a mixed moderate stenosis/regurgitation condition as Stage 2RS. Some patients in this Stage 2 SVD with symptoms could be considered for re-intervention. The most severe stage of SVD, classified as Stage 3, is the development of severe stenosis and/or regurgitation.
Indications for the Valve-in-valve Procedure
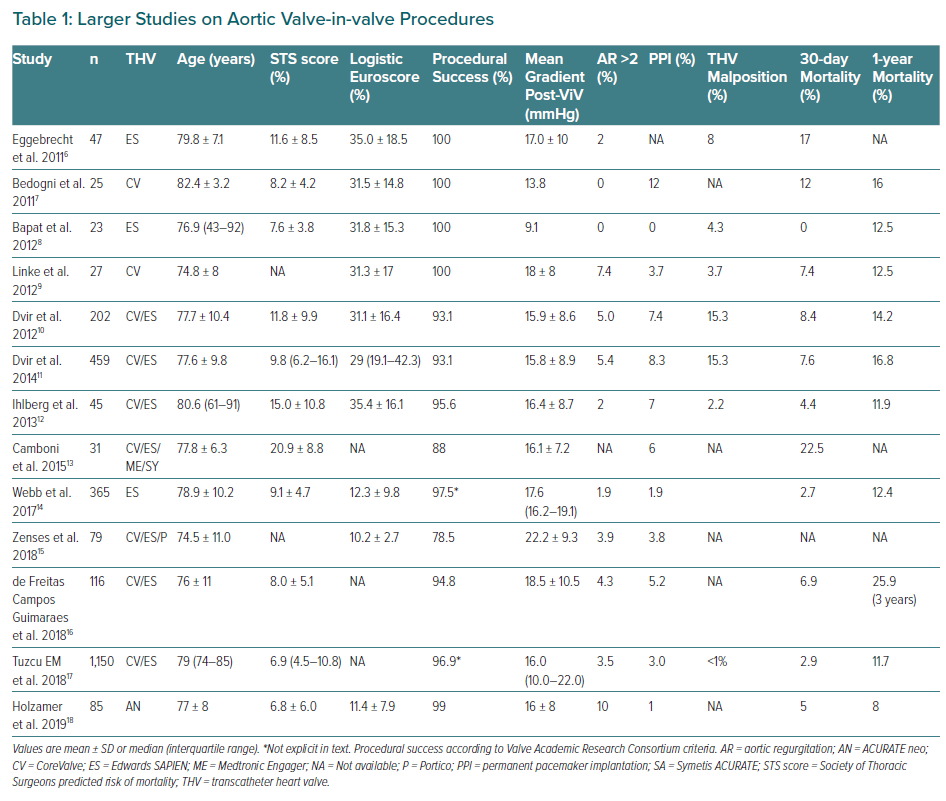
Until last decade, the standard of care for degenerated bioprosthesis (SVD Stage 3) was redo valve replacement. Due to its less invasive and appealing nature to both patients and physicians compared with redo open-heart surgery, ViV procedure rates continue to grow rapidly, even without CE mark approval for some of the current devices.5 Relatively small series and certain long registries of use of the device have been published and the findings of those with larger populations are summarised in Table 1.6–18
Tips and Tricks for Valve-in-valve Procedures
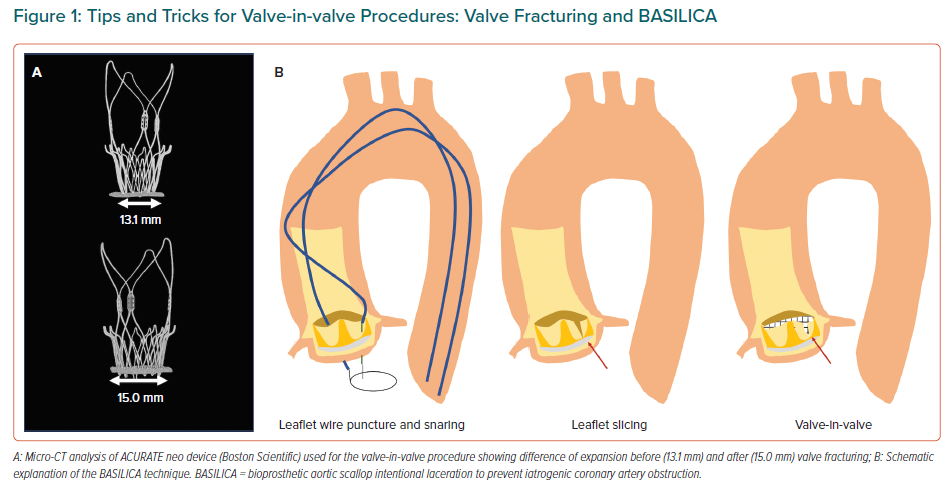
Positioning during ViV procedures can be very challenging as it is predicting the risk of coronary obstruction, more likely when the leaflets are sutured outside the sewing ring or in stentless valves.19 To facilitate better outcomes, better devices and dedicated techniques are rapidly developing to help operators. These include fracturing the ring during post-dilation to improve the transvalvular gradients in patients with prior small bioprosthesis and a certain degree of miss-match, along with the BASILICA technique (bioprosthetic or native aortic scallop intentional laceration to prevent coronary artery obstruction).20,21 These procedures are based on short series of cases but are rapidly extending in light of the promising results (Figure 1).
A relatively new scenario – likely to soon become more common – is the TAVI-in-TAVI procedure. Little is known about the mid- and long-term durability of transcatheter aortic valves beyond the first decade of implantation.22 Although the transcatheter ViV procedure is now recognised as a good alternative to redo surgery in high-risk patients with failed surgical bioprostheses, there are specific risks of TAVI-in-TAVI that differ across each device. On the one hand, supra-annular self-expandable valves might present an increased risk of coronary occlusion if treated with the current devices and render the challenging access to the coronary ostia afterwards even more difficult. This is discussed in more detail in the ‘New Implantation Strategies for TAVI’ section, below. On the other hand, intra-annular devices might obtain worse residual gradients after ViV or have an increased risk of annular rupture if post-dilation is performed. Globally, the available data on this very new scenario – albeit scarce – appear favourable.
TAVI for Bicuspid Aortic Stenosis
Incidence and Specific Challenges of Bicuspid Aortic Stenosis
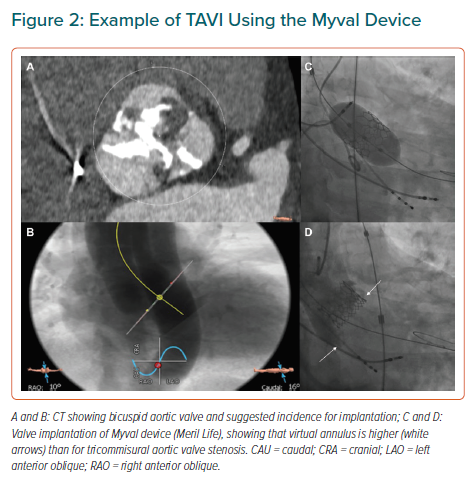
Bicuspid aortic valve (BAV) is the most common congenital valvular defect, occurring in 1–2% of the general population.23 BAV stenosis has been considered an anatomical challenge for TAVI for several reasons. First, there is often an extreme elliptical shape of the annulus and trend to aortic dilation – as opposed to the characteristic oval shape of the annulus in calcified tricuspid aortic valve (TAV) – that might be associated with greater leakage. Second, BAVs usually have higher point of coaptation of the cusps (Figure 2) that can result confounding during the procedure and increase the risk of valve embolisation. Finally, asymmetric distribution of calcium with a trend to bulky formations increases the risk of paravalvular leak and annulus rupture.24 All these elements have to be taken into account when TAVI is considered for a patient with BAV. Misdeployment is more frequent in patients with these abnormalities and might be associated with a higher rate of paravalvular regurgitation, prosthesis dysfunction or early degeneration of the implanted valve.25
Current Evidence
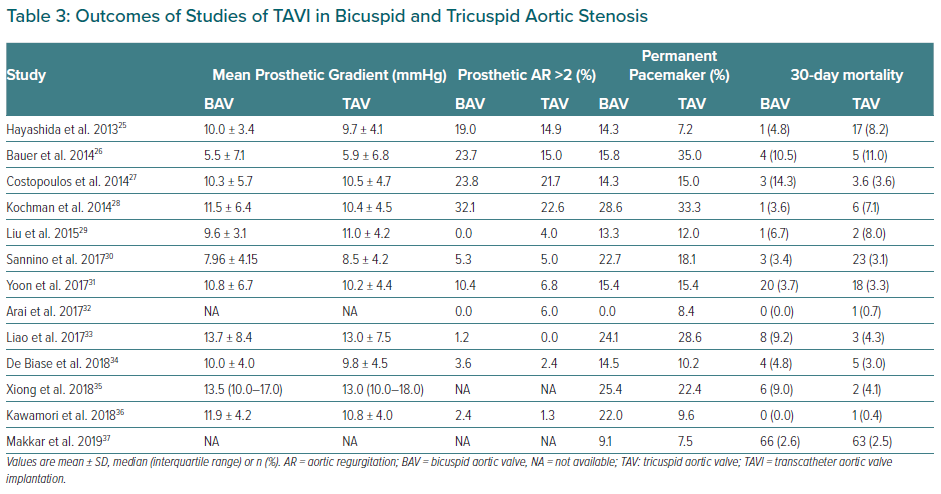
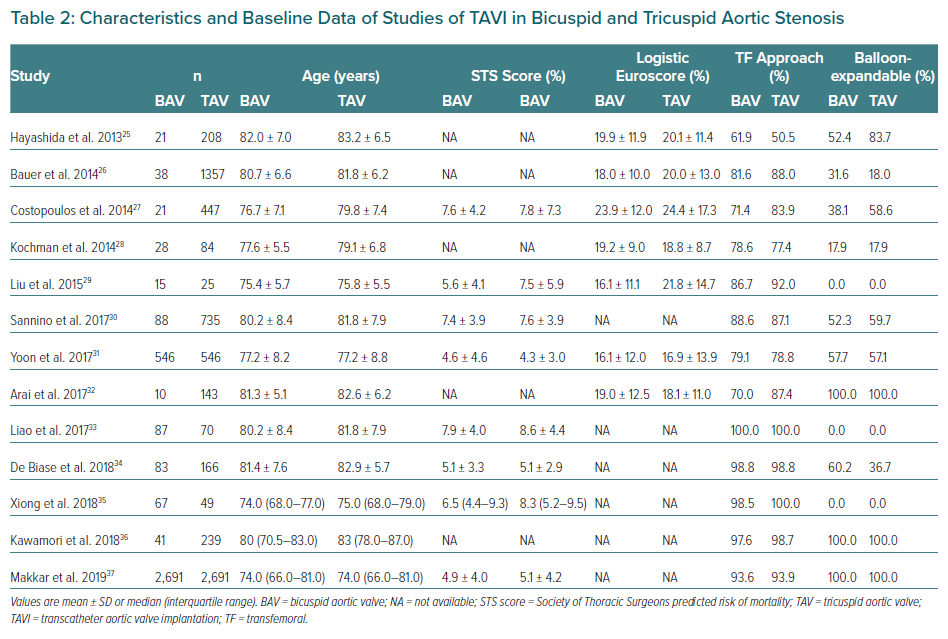
BAV patients have not been included in landmark trials of TAVI devices. To date, all specific studies analysing the different outcomes of TAVI in BAV and TAV patients are retrospective. Studies demonstrating the feasibility and safety of TAVI in BAV stenosis along with main baseline characteristics and procedural outcomes are summarised in Tables 2 and 3.26–37 Quintana et al. conducted a meta-analysis of studies mainly focused on early-generation devices.38 The analysis demonstrated that TAVI therapy was feasible and safe in BAV disease. The primary endpoint of 1-year all-cause mortality revealed 11.8% mortality in BAV compared with 15.06% in TAV patients, with no differences between groups (RR 1.03; 95% CI [0.70–1.51]). However, the BAV group was associated with a decrease in device success and an increase in significant prosthetic valve regurgitation after TAVI compared to patients with TAV.
Yoon et al. compared procedural and clinical outcomes in patients with BAV versus TAV including newer generation devices.39 Within the group receiving early-generation devices, BAV patients had more frequent aortic root injury (4.5% versus 0.0%; p=0.015) when receiving the balloon-expanding device and moderate-to-severe paravalvular leak (19.4% versus 10.5%; p=0.02) when receiving the self-expanding devices. However, among patients with newer generation devices, procedural results were comparable across different prostheses. Cumulative all-cause mortality rates at 2 years were comparable between bicuspid and tricuspid AS (17.2% versus 19.4%; p=0.28). Takagi et al. performed the latest meta-analysis available to date and demonstrated lack of statistical difference in pacemaker implantation rate and in early- and mid-term mortality (RR 1.35; 95% CI [0.94–1.93] and RR 1.00; 95 [CI 0.77–1.31], respectively).40 However, the BAV group presented a significant increase in prosthetic AR compared to TAV (RR 1.42; 95% CI [1.11–1.82]). This issue was less frequent with the use of balloon-expandable devices compared to self-expandable ones. Likely for this reason, the balloon-expandable prosthesis was the preferred option in the most recent studies (Table 2).
Is TAVI the Future Gold Standard for BAV Treatment?
In agreement with the gathered data, TAVI has been demonstrated to be an excellent option for selected BAV cases. In order to extend indications, Elbadawi et al. compared TAVI and SAVR, demonstrating similar in-hospital mortality (3.1% versus 3.1%; OR 1.00; 95% CI [0.60–1.67]).41 There were no differences between TAVI and SAVR in the rates of procedural complications and early outcomes such as cardiac arrest, cardiogenic shock, acute kidney injury, cardiac tamponade or acute stroke. TAVI was associated with lower rates of acute MI, post-operative bleeding complications and a shorter length of hospital stay. Conversely, TAVI was also associated with a higher incidence of complete heart block and permanent pacemaker insertion (13.8% versus 4.6%; OR 3.32; 95% CI [2.34–4.71]; p<0.001). However, no randomised study has yet been conducted to compare these two alternatives.
TAVI for Pure Aortic Regurgitation
Clinical Course and Current Management
AR is characterised by a prolonged silent clinical course. When patients with severe AR become symptomatic, they present with congestive heart failure owing to volume overload, increased wall stress and left ventricular dysfunction.1 The anatomy in patients with native valve AR is often challenging, with dilated aortic root, dilated ascending aorta and – often – an elliptical annulus.42 Finally, patients with AR are usually referred for valve replacement at a younger age. For these reasons, SAVR is the standard therapy.1
Mechanisms of Aortic Regurgitation
Different aetiologies for AR have been described, with degenerative, congenital and rheumatic causes the more frequent. Less often, radiotherapy or healed infective endocarditis (IE) can be responsible for this condition.
Patients who have recovered after IE but with significant valve damage represent a small but challenging population, frequently with comorbidities that increase operative risk. In recent research, TAVI in this scenario was demonstrated feasible with low risk of IE relapse at 1-year follow-up, and comparable mortality rates to the TAVI procedure in alternative settings.43 However, one-quarter of the cases presented residual significant AR.
The Role of TAVI
As a result of a clinical need, inoperable or high-risk patients with AR have been treated with TAVI worldwide.44 Scarce valve calcification is considered a contraindication for TAVI since it increases risks because of poor anchoring of the device.45 Since Roy et al. published the first case series of TAVI for pure native AR, other retrospective studies have tried to increase the evidence supporting the feasibility of the procedure for this indication.46–54 As in other TAVI scenarios, pre-procedure echocardiography and multislice 3D CT should be considered mandatory. Careful examination of the annulus, sinus of Valsalva diameters, and ascending aortic diameter measurement are essential. Valve sizing should be according to perimeter and area, but frequently adequately contrasting the annulus during CT is more difficult and the dimensions of the annulus can quickly change if the procedure is not performed shortly after the imaging evaluation. Moreover, greater oversizing might be needed.
Table 4 summarises the main registries on this topic.46–54 TAVI in pure native AR with early-generation devices was associated with relatively high rates of procedural complications. Development of new-generation devices improved procedural outcomes with lower rates of need for second valve implantation or significant post-procedural AR (≥grade 2). However, recent studies suggest that a significant reduction in the degree of AR is not sufficient because post-procedural AR ≥2 remains associated with higher rates of re-hospitalisation and all-cause mortality suggesting the need for dedicated devices.
TAVI Devices for the Treatment of Aortic Regurgitation
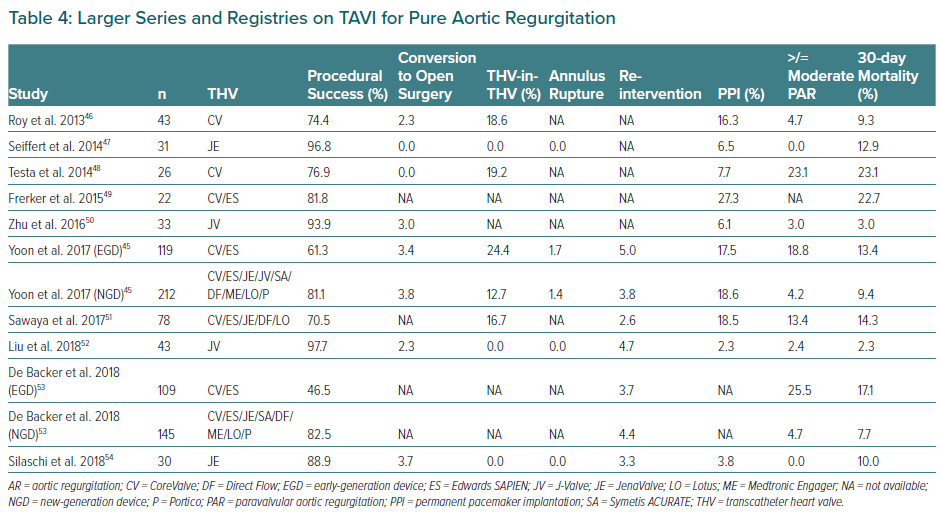
The Medtronic CoreValve (Medtronic) was preferentially chosen in early reports of TAVI for treating native pure AR. Its self-expandable properties were considered to offer stability during the implantation and ensure anchoring. However, the frequent need for a second valve and the high rates of significant post-procedural AR resulted in modest device success, as defined by the Valve Academic Research Consortium, point to the limitations of this device for the use in this setting.55 Other self-expanding transcatheter valves as ACURATE neo (Boston Scientific), Lotus (Boston Scientific), Portico (Abbott) and the balloon-expandable Edwards SAPIEN XT/S3 (Edwards Lifesciences) have been used for AR treatment (Table 4 and Figure 3) with variable outcomes but – in all cases – poorer than their results in AS patients.
Novel devices have been developed for treating patients with severe pure AR, such as the JenaValve (JenaValve Technology), the J-Valve (JieCheng Medical Technology; not available in Europe) and the Direct Flow Valve System (Direct Flow Medical), but there is still a lack of evidence to extend use for this indication.
New Implantation Strategies for TAVI
Commissural Alignment
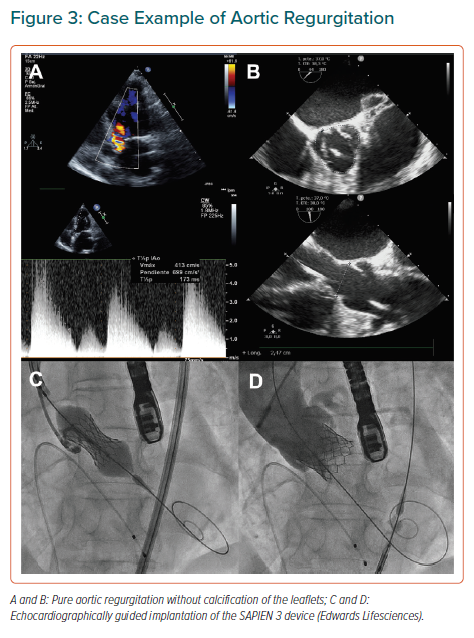
Final position of the neo-commissures is uncontrolled during transcatheter aortic valve implantation (TAVI), potentially hindering coronary access in future procedures, but also increasing the risk of coronary obstruction if a TAVI-in-TAVI procedure is eventually required due to valve degeneration. This risk is usually prevented by BASILICA technique for ViV interventions, but surgical bioprostheses are well aligned with the coronary ostia and therefore tearing the prosthesis leaflet is enough to avoid its occlusion since the tear is always in front of the ostium. On the contrary, until recently, TAVI devices were not aligned, which means that, even after BASILICA, if a new TAVI is implanted within a former degenerated one with the coronary ostia close to the commissural posts, the risk of coronary obstruction is still high after leaflet tearing. Such positioning might can make engaging the coronaries with a catheter particularly challenging, especially if a TAVI device extending into the ascending aorta has been implanted.
Different strategies have been developed to optimise coronary alignment, the great majority of them focused on rotation of the delivery system inside the vascular anatomy, which might lack of accuracy and is not risk-free due to intravascular manipulation of the device.56,57 More recently, a new strategy for self-expandable devices based on the estimation of a patient-specific rotation of the delivery system before introducing it into the patient has been described.58
This strategy is based on CT analysis with a specific software and can be complemented by the use of a dedicated tool designed for precise measurement of the exact number of degrees that the delivery system is rotated (Figures 4A and 4B). Furthermore, the concept can be applied to balloon-expandable devices by crimping the valve with the commissure in a specific position and advancing the delivery system as recommended by the manufacturer (Figure 4C).
Optimal Valve Implantation Depth
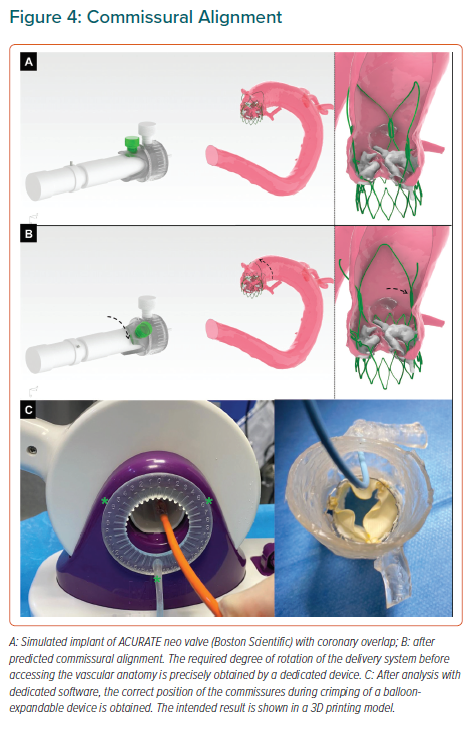
Since description of the technique, the vast majority of operators have used the three-cusp coplanar view, where the three aortic cusps are angiographically aligned, having the right coronary cusp (RCC) between the noncoronary cusp (NCC) and the left coronary cusp (LCC). However, description of cusp-overlap view, comprising overlapping the RCC and LCC (Figure 5) has been associated with a significant reduction in pacemaker rates with self-expanding prostheses. First, this projection eliminates parallax of the delivery catheter and presents the delivery catheter more centred across the aortic valve. Second, and most important, the en-face view of NCC allows higher deployment – reducing conduction disturbances – and simultaneously minimising the risk of device aortic displacement, especially in large annuli with minimal oversizing.59
Recently, Ben-Shoshan et al. compared the double S-Curve technique versus cusp-overlap view.60 This method is based in automated software calculations of optimal projection, which is an S-shaped curve of continuous pairs of C-arm angulations orthogonal to the axial plane of the aortic valve annulus. The second S-curve is the delivery catheter calculation. At the intersection point of these two curves, both delivery catheter and aortic annulus are perpendicular amongst them without foreshortening. Both cusp-overlap and double S-curve models provide right and caudal projections in most cases (Figure 5).
Conclusion
The TAVI procedure is rapidly expanding in use, but limited evidence exists for certain challenging indications. The ViV procedure is still not fully established, although it has become the preferred alternative for failing bio-prostheses. In order to obtain optimal outcomes, operators need to master several complex techniques such as valve fracturing and BASILICA. TAVI for BAV stenosis might be a good alternative in terms of mortality and major complications but has not been demonstrated superior to surgery. If TAVI is decided, the best device to use remains unclear since balloon-expandable prosthesis might have slightly higher rate of annular rupture and self-expanding devices a greater rate of pacemaker and paravalvular leak. Finally, there is a clear need for new devices to improve the results of TAVI for the treatment of AR. Until then, TAVI should be considered only in prohibitive risk patients and after careful imaging evaluation.
Novel devices along with optimised implantation strategies will increase success rates with the TAVI procedure, reducing complications such as valve embolisation and conduction disturbances and simplifying future interventions in patients harbouring percutaneous valves.