AF is the most common sustained cardiac dysrhythmia encountered in clinical practice.1 Treatment strategies involve antiarrhythmic drug therapy and catheter ablation targeting the pulmonary veins (PVs) and other arrhythmogenic sites.2 However, these therapies have limitations and the effect of ablation on hard endpoints remains unclear. For patients with persistent AF in particular, the results of pulmonary vein isolation (PVI) are currently suboptimal. Therefore, there is growing interest in pursuing alternative therapies particularly in the setting of persistent AF able to overcome the modest results of PVI.
In this review we seek to describe evolving alternative strategies for the management of AF, including non-invasive and percutaneous autonomic modulation. Approaches to achieve this modulation include tragus stimulation, renal denervation, cardiac afferent denervation, alcohol injection in the vein of Marshall (VOM), baroreceptor activation therapy, endocardial ganglionated plexi (GP) ablation and percutaneous stellate ganglion block (PSGB; Table 1). Although promising, these therapies are currently still under investigation. Nevertheless, they may become part of the treatment of AF in combination with conventional PVI in the near future.
Autonomic Pathways and Ganglionated Plexi
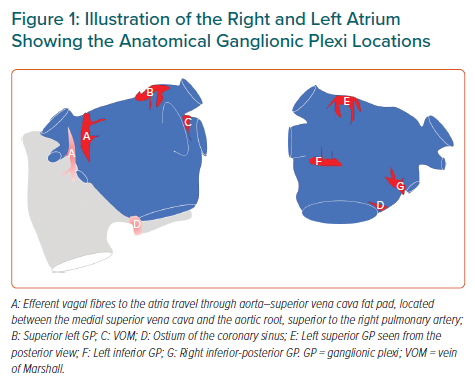
The heart is innervated by the extrinsic (central) and the intrinsic cardiac autonomic nervous system (ANS). The extrinsic ANS consists of the ganglia in the brain or along the spinal cord, where the cell bodies reside, as well as their axons en route to the heart. The intrinsic ANS is comprised of an extensive epicardial neural network of nerve axons, interconnecting neurons and clusters of autonomic ganglia known as GP not only on the atria, but also on both ventricles.3–4 These GP, most of which are embedded within epicardial fat pads, vary in size from those that contain just a few neurons to those that contain over 400 neurons. Notably, the highest density of autonomic innervation is found at the posterior wall of the left atrium (LA), particularly at the PV–atrial junction. Several studies have demonstrated that the GP are composed of a heterogeneous population of neurons, including efferent, afferent and interconnecting neurons – the latter group comprising the majority of the neural elements within the GP. From a physiological point of view, GP contain both sympathetic and parasympathetic elements. Importantly, the GP serve as the communication centres between the intrinsic and extrinsic central autonomic nervous system, coordinating the response to afferent and efferent neural trafficking to control regional electrophysiological, vascular and contractile function. Anatomically, the four major atrial GP are located in close association with the PVs and each innervate one of the four PVs, as well as the surrounding atrial myocardium. The Oklahoma group renamed the major atrial GP.5 The anterior right GP is located immediately anterior to the right superior PV – often extending inferiorly – to the region anterior to the right inferior PV. The superior left GP is located at the roof of the LA, 1–2 cm medial to the left superior PV. The right and left inferior GP are located at the inferior aspect of the posterior wall of the LA, 2–3 cm below the right and left PVs, respectively (Figure 1). The ligament of Marshall (LOM), located between the left atrial appendage and the left superior PV, also contains autonomic neurons.
Several studies have demonstrated that the ANS and the epicardial GP play a central role in the initiation and maintenance of AF, especially in the early stages.6–9 This makes autonomic modulation an attractive modality for AF therapy. Such modulation can be achieved using a variety of approaches as described in the following sections.
Neuromodulatory Approaches
Low-level Tragus Stimulation
Studies in dogs have demonstrated that vagal nerve stimulation (VNS) via direction stimulation of the cervical vagus nerve at voltages significantly below those resulting in sinus rate and atrioventricular conduction slowing is able to suppress AF inducibility and shorten AF duration.10–13 Based on the fact that the auricular branch of the vagus nerve has communication with the skin of the tragus, transcutaneous stimulation of the vagus nerve at this site has been entertained as a possible method of non-invasive vagal stimulation.14 Importantly, it seems that tragus stimulation preferentially activates afferent rather than efferent vagal fibres, which may offer a therapeutic advantage.15 Specifically, tragus stimulation has been shown to activate central vagal projections in the brain in humans, leading to decreased sympathetic output.16 Additionally, tragus stimulation – as opposed to cervical VNS – may avoid concomitant stimulation of sympathetic fibres, which are co-localised with vagal fibres in the vagus nerve and are inadvertently stimulated during cervical VNS in humans.17 Importantly, tragus stimulation may avoid the adverse effects reported with cervical VNS, including cough, nausea, dysphonia and tinnitus.18
Yu et al. performed tragus stimulation in a rapid atrial pacing (RAP) canine model of AF via alligator clips attached directly to the tragus, at 80% of the bradycardia threshold. RAP induced an initial progressive decrease in effective refractory period (ERP), increase in AF inducibility and increase in neural activity from the anterior right GP, all of which were significantly attenuated by subsequent tragus stimulation. Bilateral vagal transection distal to the stimulation site eliminated the ability of tragus stimulation to reverse the effects of RAP on ERP and AF inducibility, confirming the mechanism of tragus stimulation is mediated by the efferent vagus nerve.19
In a human study, Stavrakis et al. randomised 40 patients presenting for ablation of paroxysmal AF to either tragus stimulation or sham stimulation (attachment of the stimulating electrodes with no energy delivery) prior to ablation (frequency 20 Hz; 50% of bradycardia threshold). AF was induced by RAP before and after tragus stimulation (or sham) to assess the effect of stimulation on duration of pacing-induced AF and number of pacing attempts required to induce AF. They reported a significant reduction in pacing-induced AF duration at 1 hour (10.4 ± 5.2 minutes versus 18.5 ± 5.6 minutes; p=0.002) in the active but not in the sham group, number of burst pacing attempts required to induce AF, as well as increases in the ERP recorded from the right atrium and LA.20
In the TREAT-AF study, Stavrakis et al. randomised 53 patients to tragus stimulation or sham for 1 hour daily over a 6-month period after individual training on device use. After adjusting for baseline values, AF burden at 6 months was 85% lower in the active group compared to sham, with a concomitant 23% reduction in tumour necrosis factor α levels.21 These results are particularly promising because they show that a self-administered treatment with essentially no significant risks can have a significant impact on AF burden and levels of systemic inflammation.
Lastly, this approach appears to be paradoxical, especially in the context of vagally mediated AF. However, in a canine model, there was no increase in AF inducibility until VNS significantly slowed the heart rate.22 In summary, GP ablation has been evaluated in animal models and human subjects and shown to eliminate vagal response and subsequently abolish AF. Nevertheless, more data will be needed in this particular setting.
Renal Denervation
The peri-renal abdominal aorta and proximal renal arteries are associated with a rich network of sympathetic ganglia and nerve fibres that provide afferent and efferent signalling between the central nervous system (CNS) and the abdominal and pelvic viscera.23 The beneficial effect of renal denervation in AF patients is thought to be related to attenuation of afferent sympathetic input from the aorticorenal ganglion (ARG) to the CNS, as well as attenuation of efferent signalling from the ARG to the renal parenchyma, reducing renin–angiotensin–aldosterone system activation. These effects would be expected to reduce both the electrophysiological and structural remodelling that contributes to the initiation and maintenance of AF.23
Pokushalov et al. randomised 27 patients with drug-refractory AF and resistant hypertension to PVI alone versus PVI with renal denervation. The renal denervation was performed by making discrete lesions (8–10 W for 2 minutes) beginning at the first major renal artery bifurcation tracking longitudinally back to the main artery ostium.24 This technique was repeated rotationally to cover the circumference of the artery. There was a significantly greater freedom from AF in the PVI + renal denervation group compared with the PVI only group (69% versus 29%; p=0.033).
In a prospective pilot cohort study, Feyz et al. performed renal denervation in 20 patients with paroxysmal AF and resistant hypertension after placement of an implantable cardiac monitor. The daily AF burden was significantly reduced from median 1.39 minutes (interquartile range: 0–10.9) before renal denervation to median 0.94 minutes (interquartile range: 0–6.0) at 12 months (p=0.03) and there was a positive effect on quality of life.25 With these encouraging results, the ERADICATE-AF study was performed as the first large, multicentre trial to assess the effect of renal denervation on freedom from AF without therapy when added to standard PVI.26 A total of 302 patients with paroxysmal AF and hypertension receiving at least one anti-hypertensive drug were randomised to PVI or PVI + renal denervation. At 12 months, significantly more patients who underwent renal denervation were free from AF off therapy compared with controls (71.4% versus 57.8%; HR 0.61; 95% CI [0.41–0.90]; p=0.011). Of note, seven patients in each group had a complication: 4.7% in the isolation-only group and 4.5% in the renal denervation group for an absolute risk difference of 0.1% (95% CI [−4.0, 4.4%]; p>0.99). There were no renal artery or femoral artery complications. All complications in both groups were attributed to the PVI procedure.
Cardiac Afferent Denervation
It seems well defined that sensory neurons within the GP play a role in the mechanistic origin of AF. Moreover, sensory neurons are very relevant in the relationship between apnoea and AF. Obstructive sleep apnoea syndrome has been strongly associated with AF, affecting its prevalence and the outcomes of PVI.27 However, the ANS response to apnoea and its mechanistic connection to AF are unclear.
Our group aimed to shed light on the autonomic response to apnoea and to test the effects of ablation of cardiac sensory neurons using the neurotoxic transient receptor potential vanilloid 1 (TRPV1) agonist resiniferatoxin (RTX) in an animal model.28 Sixteen dogs were anaesthetised and ventilated with apnoea induced by stopping ventilation until oxygen saturations decreased to 80%. Nerve recordings from bilateral vagal nerves, left stellate ganglion and anterior right GP were obtained before and during apnoea. The atrial ERP and AF inducibility on single extrastimulation were assessed before and during apnoea, and before and after intrapericardial RTX administration. Immunohistochemical staining for TRPV1 was performed on GPs.
Apnoea increased anterior right GP activity, followed by clustered crescendo vagal bursts synchronised with heart rate and blood pressure oscillations. On further oxygen desaturation, a tonic increase in stellate ganglion activity and blood pressure ensued. Apnoea induced ERP shortening from 110.20 ± 31.3 ms to 90.6 ± 29.1 ms (p<0.001) and AF was induced in all nine dogs versus none of the nine at baseline. After RTX administration, increases in GP and stellate ganglion activity and blood pressure during apnoea were abolished, ERP increased to 126.7 ± 26.9 ms (p<0.001) and AF was not induced. Vagal bursts remained unchanged. GP cells showed cytoplasmic microvacuolisation and apoptosis. This led us to conclude that apnoea increases GP activity, followed by vagal bursts and tonic stellate ganglion firing. RTX decreases sympathetic and GP nerve activity, abolishes apnoea’s electrophysiological response and AF inducibility. Subsequently, this suggests that sensory neurons mediate the electrophysiological response to apnoea and could be a valid therapeutic target to reduce apnoea-induced AF. Since chronically repeated obstructive sleep apnoea episodes have been shown to cause cardiac remodelling with fibrosis playing a prominent role contributing to AF promotion, this mechanistic study could open the door to future therapies that investigate the chronic effects of chemical ablation of cardiac GP sensory neurons in the setting of AF ablation procedures. Moreover, sensory neurons mediate the electrophysiological response to apnoea and could be a valid therapeutic target to reduce apnoea-induced AF.
Vein of Marshall Ethanol Infusion
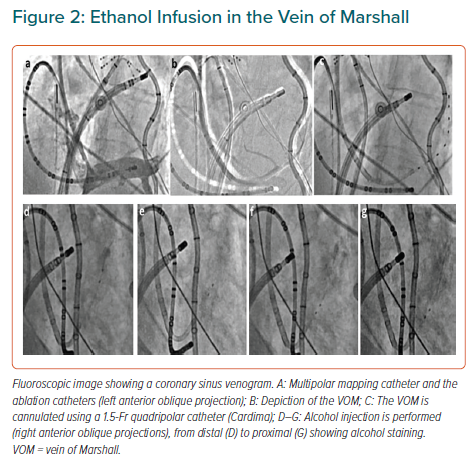
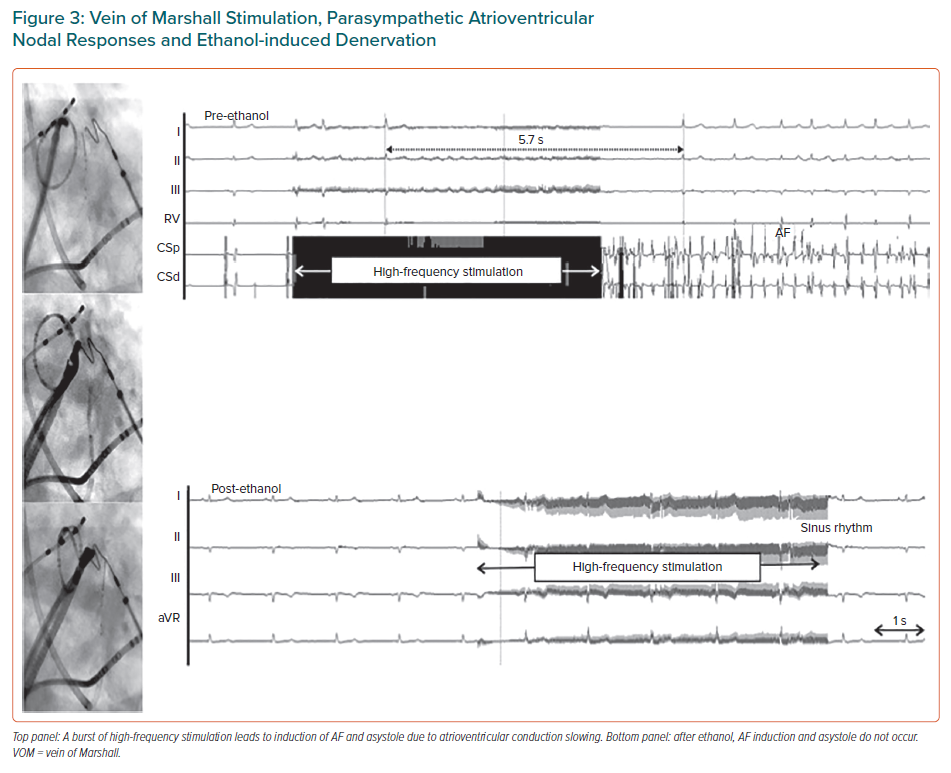
The VOM is an embryological remnant of the left superior vena cava. It has been implicated in the pathogenesis of AF, as a source of AF triggers and as a tract for parasympathetic and sympathetic innervations that modulate electrophysiological properties of atrial tissue and contribute to AF maintenance.29–32 The LOM can be conceived as a connecting pathway between intrathoracic cardiac ganglia and intrinsic cardiac ganglia, specifically the inferior left ganglion, which is located below the left inferior PV and would coincide more closely with the VOM as it connects with the coronary sinus. It is also important to recognise that the anatomical location of the LOM–VOM in the LA ridge coincides with the ventrolateral cardiac nerve, destined to provide innervation to the posterolateral left ventricle. We showed that in humans the VOM and its neighbouring atrial myocardium contain intracardiac nerves that can reach the atrioventricular node and induce parasympathetic responses and that this response can be abolished by ethanol infusion in the VOM (Figures 2 and 3).32 Additionally, the VOM is located within the mitral isthmus, critical for perimitral atrial tachycardia (AT).31 Retrograde balloon cannulation and ethanol infusion in the VOM create a local ablation, eliminate AF triggers and VOM innervation, facilitate mitral isthmus ablation, and have shown potential in preliminary studies.32–34
The VENUS trial tested the hypothesis that adding VOM ethanol infusion to de novo catheter ablation of persistent AF could lead to increased chances of maintaining normal rhythm.33 This was a multicentre, randomised clinical trial comparing the rhythm-control effectiveness of two ablation strategies: catheter ablation alone or combined with VOM ethanol infusion in de novo ablation of AF. The primary outcome was freedom from AF or AT lasting longer than 30 seconds after the performance of a single procedure (VOM–catheter ablation or catheter ablation alone), without the use of antiarrhythmic drugs and occurring after the blanking period, including monitoring at 6 and 12 months. Excluding patients in whom VOM ethanol infusion was not performed (as-treated analysis), the primary outcome was reached in 80 of the 155 patients in the VOM–catheter ablation group (51.6%; p=0.02), with an absolute difference of 13.6% (95% CI [2.7–24.6%]) and OR 0.57 (95% CI [0.37–0.90]).
Differences in the primary outcome were driven by a reduced recurrence of AF or AT in the VOM–catheter ablation group. Kaplan–Meier plots showed significant reduction in AF or AT recurrence in the VOM–catheter ablation group, both in the as-randomised analysis (HR 0.73; 95% CI [0.53–1.00]; p=0.05) and the as-treated analysis (HR 0.67; 95% CI [0.47–0.93]; p=0.02). At 6 and 12 months, freedom from any AF or AT on 1-month monitoring (zero burden), after repeat procedures, or with the use of antiarrhythmic drugs was achieved in 67.8% of monitoring sessions (184/271) in the catheter ablation group, in 75.8% (232/306) in the VOM–catheter ablation group (absolute difference 8%; 95% CI [0.6–15.2]; p=0.03), and in 78.3% of those undergoing successful VOM ethanol infusion procedures (206/263) (as treated: absolute difference 10.5%; 95% CI [2.9–17.9]; p=0.008). In the subgroup analyses, female sex, longstanding persistent AF and LA volume >75 ml/m2 were associated with a greater effect in the VOM–catheter ablation group. Importantly, in the VENUS trial, ethanol infusion procedure did not increase procedural complications.
The most common intraprocedural adverse events were vascular access complications (haematoma or pseudoaneurysm, 11 events) and intraprocedural pericardial effusion (three events). After the procedure, subacute pericardial effusion requiring pericardiocentesis occurred in four patients. There were seven patients with cerebrovascular events and seven with pneumonia after the procedure. Symptomatic inflammatory pericarditis not requiring drainage occurred in 11 patients in the VOM–catheter ablation and in six in the catheter ablation group. Fluid overload requiring diuretic treatment occurred in 10 patients in the VOM–catheter ablation group and in two patients in the catheter ablation group.
Derval et al. examined a comprehensive ablation strategy comprising Marshall bundle elimination, PVI and line completion for anatomical ablation of persistent AF (Marshall-PLAN) that was strictly based on anatomical considerations.35 At 12 months, in the overall cohort 54 of 75 patients (72%) were free from AF/AT after a single procedure (no antiarrhythmic drugs). In the subset of patients with a complete Marshall-PLAN lesion set (n=68), the single procedure success rate was 79%. After one or two procedures, 67 of 75 patients (89%) remained free from AF/AT (no antiarrhythmic drugs). After one or two procedures, VOM ethanol infusion was complete in 72 of 75 patients (96%). The authors concluded that this approach is feasible, safe and associated with a high rate of freedom from arrhythmia recurrence at 12 months in patients with persistent AF.
All in all, these strategies could be superior to linear lesions or ablation of complex potentials, strategies that have failed to improve the outcome of PVI in previous randomized trials.36
Baroreceptor Activation Therapy
Carotid baroreceptor stimulation (BRS) is another strategy to modulate autonomic balance by activation of the baroreflex. While its effects on heart failure and blood pressure are currently under investigation, the effects of BRS on atrial electrophysiology and arrhythmias are unclear. BRS modulates autonomic balance not just by sympathetic withdrawal but also by increased vagal activation, finally resulting in reduced total body sympathetic drive. Reduced sympathetic drive is generally considered to result in an atrial antiarrhythmic effect.37–38 However, increased vagal tone, as also associated with BRS, could potentially shorten atrial refractoriness thereby increasing the vulnerable phase and resulting in the stabilisation of reentry circuits perpetuating AF, comparable to vagal stimulation.
Linz et al. observed that BRS, at a stimulation intensity slowing heart rate and reducing blood pressure similar to that described in hypertensive patients, resulted in a shortening of atrial refractoriness and in a pronounced increase in AF inducibility in pigs. This was mediated by an increase in vagal tone, as it was attenuated by atropine.37 Dai et al. investigated the effects of low-level BRS (LL-BRS) on AF in three acute dog models: AF induced by 6 hours of rapid atrial pacing, AF induced by acetylcholine injected into the anterior right GP and AF induced by acetylcholine applied to the right atrial appendage.39 LL-BRS could reverse rapid atrial pacing-induced atrial electrical remodelling. This was associated with a reduction in sympathetic activation as indicated by decreased activation of the left stellate ganglion, lower plasma norepinephrine levels and reduced low-frequency/high-frequency ratios describing heart rate variability. In addition, acetylcholine-induced AF could be suppressed. They concluded that the inhibition of the left stellate ganglion activity is likely to contribute to the demonstrated antiarrhythmic effects in different AF models. In addition to these observations, LL-BRS has been previously demonstrated to inhibit GP activation.40
These data suggest that both extrinsic and intrinsic cardiac autonomic systems are modulated by LL-BRS underlying the antiarrhythmic effects of LL-BRS.40 It could indicate that as the effects of vagal activation by BRS may be dependent on the prevailing sympathetic drive, the levels of BRS necessary to reduce blood pressure and heart rate and to shorten atrial refractoriness may be different in normotensive subjects compared with hypertensive subjects. Further studies are necessary to explore the optimal stimulation intensity of BRS to avoid atrial proarrhythmic side effects and to safely display antiarrhythmic effects in patients with AF.
Endocardial Ganglionated Plexi Ablation
PV myocytes have shorter action potential duration and a greater sensitivity to both cholinergic and adrenergic stimulation than adjacent atrial tissue, which may explain why AF usually starts with an extra beat arising from PVs.41–45 Figure 1 depicts the anatomical location of GP. To define the mechanism of how a single PV depolarisation is converted to AF, Scherlag et al. investigated the effects of GP stimulation at voltages ranging from 0.6 to 4.0 V in 14 anaesthetised dogs.41 They demonstrated that stimuli applied to PVs would not induce AF unless there was simultaneous stimulation of the adjacent GP (20 Hz, 0.1 ms pulse width) that excites the atrial myocardium. The same group showed that muscarinic receptor blockade prevented action potential duration shortening and focal firing originating from the adjacent PV.42
Furthermore, Choi et al. demonstrated that activation of intrinsic cardiac ANS is observed prior to the onset of paroxysmal AF in nearly 100% cases, where 20% suffered the attack in the absence of extrinsic cardiac ANS afferent signals, suggesting that intrinsic part or GPs could trigger AF completely independently of extrinsic ones.6 Subsequently, since conventional PVI transects three of the four major atrial GP as well as the LOM, it is possible that in the clinical setting, autonomic denervation caused by PVI might play a central role in the efficacy of PVI. On the other hand, PV based approaches may not be enough for vagal denervation and more selective localisation of GPs during electrophysiological study may improve the results. This approach – neuromodulation through GP ablation in combination with PVI – has been investigated in recent years.
In the first human study, Pappone et al. evaluated the potential role of GP ablation by radiofrequency in preventing recurrent AF in patients undergoing circumferential PVI for paroxysmal AF.46 Katritsis et al, performed a randomised study comprising 242 patients with symptomatic paroxysmal AF. They were randomised to treatment as follows: circumferential PVI (n=78), anatomic ablation of the main left atrial GP (n=82), or circumferential PVI followed by anatomic ablation of the main left atrial GP (n=82).47 Freedom from AF or AT was achieved in 44 (56%), 39 (48%), and 61 (74%) patients in the PVI, GP, and PVI + GP groups, respectively (p=0.004 by log-rank test). The PVI GP ablation strategy compared with PVI alone yielded HR 0.53 (95% CI [0.31–0.91]; p=0.022) for recurrence of AF or AT. No serious adverse procedure-related events were encountered.
Pokushalov et al. randomised 264 patients with long-lasting persistent (32%) and persistent AF (68%) to PVI GP ablation (n=132) or PVI + linear lesions (LL; n=132).48 By using an implantable monitoring device, they identified that after 3 years, 34% of patients with PVI + LL and 49% of patients with PVI+GP ablation, respectively, maintained sinus rhythm (p=0.035). After a second procedure, long-term success was seen in 52% of the PVI + LL group and in 68% of the PVI + GP group, respectively (p=0.006).49
A main limitation of the study was that techniques were not compared with a PVI-alone strategy. However, due to the feasibility of the technique at the time of PVI and the experience gained for the treatment of patients with neutrally-mediated syncope and severe cardioinhibitory response, this approach might be an alternative not only to increase the success rate but also for those patients with sinus node dysfunction.
Of note, some other studies have not shown any statistically significant differences in AF recurrence rates between the study groups with a not insignificant risk of potential complication, such as sinus node dysfunction requiring pacemaker implantation.50 Further studies are urgently needed before the generalisation of the technique. In our experience we have only performed it in exceptional situations.
Percutaneous Stellate Ganglion Block
PSGB provides an alternative and effective non-pharmacological approach to controlling cardiac arrhythmias. The procedure can be performed at the bedside thorough a minimally invasive approach guided by ultrasonography. It has shown a potential benefit in the setting of AF and ventricular arrhythmias. For instance, ablation of bilateral stellate ganglia was shown to eliminate AT episodes in a canine model of pacing-induced heart failure.51
A pilot clinical study of 36 patients undergoing PVI, as well as short duration PSGB (unilateral, left or right) demonstrated lengthened atrial ERP while reducing inducibility and duration of AF.52 With regard to ventricular arrhythmias, Tian et al. evaluated the role of PSGB in 30 consecutive patients with drug-refractory electrical storm. At 24 hours, 60% of patients were free from ventricular arrhythmias.53 Importantly, patients whose ventricular arrhythmias were controlled had a lower hospital mortality rate than patients whose arrhythmias continued (5.6% versus 50.0%; p=0.009).
Conclusion
The understanding of the mechanistic aspects of AF remains incomplete. This may be one of the reasons underlying the suboptimal results of current available therapies. Among potential strategies, autonomic modulation remains a promising strategy, and several approaches for this modulation have been proposed. Larger randomised controlled studies are now needed to better define the subset of AF patients who would benefit most from catheter based autonomic modification, along with the best approach to achieve it and the effect on the success rate.