Obesity has nearly tripled since 1975, and the WHO estimates that in 2016 more than 1.9 billion (39%) adults worldwide were overweight and 650 million (13%) were obese.1 In the UK, this epidemic is perpetuated and, in 2018, 60% of women in England were overweight or obese and 27% were obese.2 By 2030, it is estimated that there will be 11 million more adults with obesity in the UK, costing around £1.9–2 billion per annum.3 Obesity significantly impacts pregnancy and is associated with marked maternal and perinatal morbidity and mortality.4 Maternal cardiac function plays a crucial role in normal pregnancy, as well as in the pathophysiology of complicated pregnancies.5,6 The aim of this review is to describe the available evidence on maternal cardiac function in pregnancies affected by obesity, gestational diabetes (GDM) and hypertension, as well as in pregnancies in women with previous bariatric surgery.
Obesity is becoming increasingly prevalent in the UK and affects 21.3% of the antenatal population. Furthermore, fewer than half of pregnant women (47.3%) have a BMI within the normal range.7 Obesity in pregnancy is associated with an increased risk of miscarriage, GDM, hypertensive disorders, pre-eclampsia (PE), venous thromboembolism, induction of labour, dysfunctional/prolonged labour, caesarean section, anaesthetic complications, postpartum haemorrhage, wound infections and mortality.4,7
GDM results from impaired glucose tolerance due to pancreatic β-cell dysfunction on a background of chronic insulin resistance, with one Australian study reporting GDM in 13.8% of obese and 21.6% of morbidly obese pregnant women.8 A meta-analysis has shown that the ORs of developing GDM among overweight, obese, and severely obese pregnant women are 2.14, 3.56 and 8.56, respectively, compared with normal-weight pregnant women.9 Increases have also been reported in the prevalence of both type 1 (T1D) and type 2 (T2D) diabetes in pregnancy, with the prevalence of T1D increasing from 1.56 to 4.09 per 1,000 pregnancies between 1995 and 2015 and that of T2D increasing from 2.34 to 10.62 per 1,000 pregnancies between 1995 and 2012.10 In general, diabetes in pregnancy significantly increases the risk of preterm delivery, large for gestational age (LGA) infants and adverse pregnancy outcomes.11 For the mother, diabetes increases the risk of PE and caesarean section, and approximately 60% of those with GDM will develop T2D later in life.12,13
The prevalence of chronic hypertension is estimated at around 0.6–2.7% of all pregnancies with two main risk factors of obesity and advanced maternal age.14 With regard to hypertensive disorders that arise in pregnancy, the prevalence of gestational hypertension (GH) and PE is estimated at 4.2–7.9% and 1.5–7.7% of all pregnancies, respectively.14 Obesity increases the risk of hypertensive disorders, with the prevalence of GH reported as 10.2% in women who are obese and 12.3% in women who are morbidly obese.15 A systematic review of 52 studies reported that the risk of PE is increased by 50% in women with obesity in early pregnancy and that the risk doubles with each 5- to 7-kg/m2 increase in pre-pregnancy BMI.16,17 A 2022 report from Mothers and Babies: Reducing Risk through Audits and Confidential Enquiries across the UK (MBRRACE-UK) found that of the women who died in pregnancy and up to 1 year after, almost one-third (27%) were obese.18
Metabolic syndrome includes several known cardiovascular risk factors, including insulin resistance, obesity, dyslipidaemia and hypertension.19 Metabolic syndrome can identify those at high risk of developing cardiovascular disease and T2D in the future and has a prevalence of approximately 24.3%, which increases with advancing age.20 Metabolic syndrome, defined according to the International Diabetes Federation criteria (i.e. waist circumference ≥80 cm plus any two of the following: raised triglycerides [≥1.70 mmol/l], reduced HDL [<1.29 mmol/l], raised blood pressure [BP; defined as systolic BP ≥130 mmHg or diastolic BP ≥85 mmHg] or raised plasma glucose [≥5.6 mmol/l]).21 It has been shown that more than half the women with metabolic syndrome in early pregnancy will develop GDM or PE later.21
Unfortunately, lifestyle modifications and pharmacological interventions have failed to address the obesity epidemic. Bariatric surgery is the most successful treatment thus far for sustainable weight loss, with around 6,000 procedures performed annually in the UK.22 Over 70% of these procedures are performed in women, most of whom are of reproductive age.22 Bariatric surgery has been shown to have an effect on reproductive health because prepregnancy maternal bariatric surgery has been associated with improved fertility and a reduction in miscarriage rates, the prevalence of hypertension in pregnancy, GDM and the delivery of LGA neonates, as well as increased rates of small for gestational age (SGA) neonates and late preterm delivery.23,24
Maternal Cardiac Adaptation to Normal Pregnancy
Normal pregnancy is associated with significant maternal cardiac and haemodynamic changes to support the gravid uterus. In early pregnancy, hormonal changes lead to reduced systemic vascular resistance, vasodilation and increased vascular capacitance, resulting in an underfilled vascular system, which drives plasma volume expansion.5,25,26 In particular, oestradiol activates the renin–angiotensin–aldosterone system (RAAS), leading to increased sodium reabsorption and plasma volume expansion by almost 50%.27-29 Despite the reduction in systemic vascular resistance, there is only a small decrease in BP in the first and second trimesters, due primarily to increased cardiac output (CO), which is due to a 30% increase in stroke volume (SV) and a smaller increase in heart rate.27–29 CO is increased by 30–50%, starting from as early as 6 weeks gestation and peaking at 20–28 weeks gestation, after which it is maintained until term.27–29 There is physiological cardiac hypertrophy to cope with the increased preload; the ventricular hypertrophy increases contractility and contributes to the rise in SV. End-diastolic volume is increased via increased ventricular mass and valvular diameters, and this is despite a reduced filling time and a shorter cardiac cycle due to higher heart rate.5 Although the heart is dilated due to the increased end-diastolic volume and ventricular hypertrophy, ejection fraction (EF) is maintained.5,27 However, some studies have shown a reduction in global strain between the second and third trimesters where EF showed no significant change.30,31 With regard to diastolic function, studies have shown that the E/A ratio falls with advancing gestation, indicating a greater reliance on atrial contraction.32
Maternal Cardiac Function and Obesity
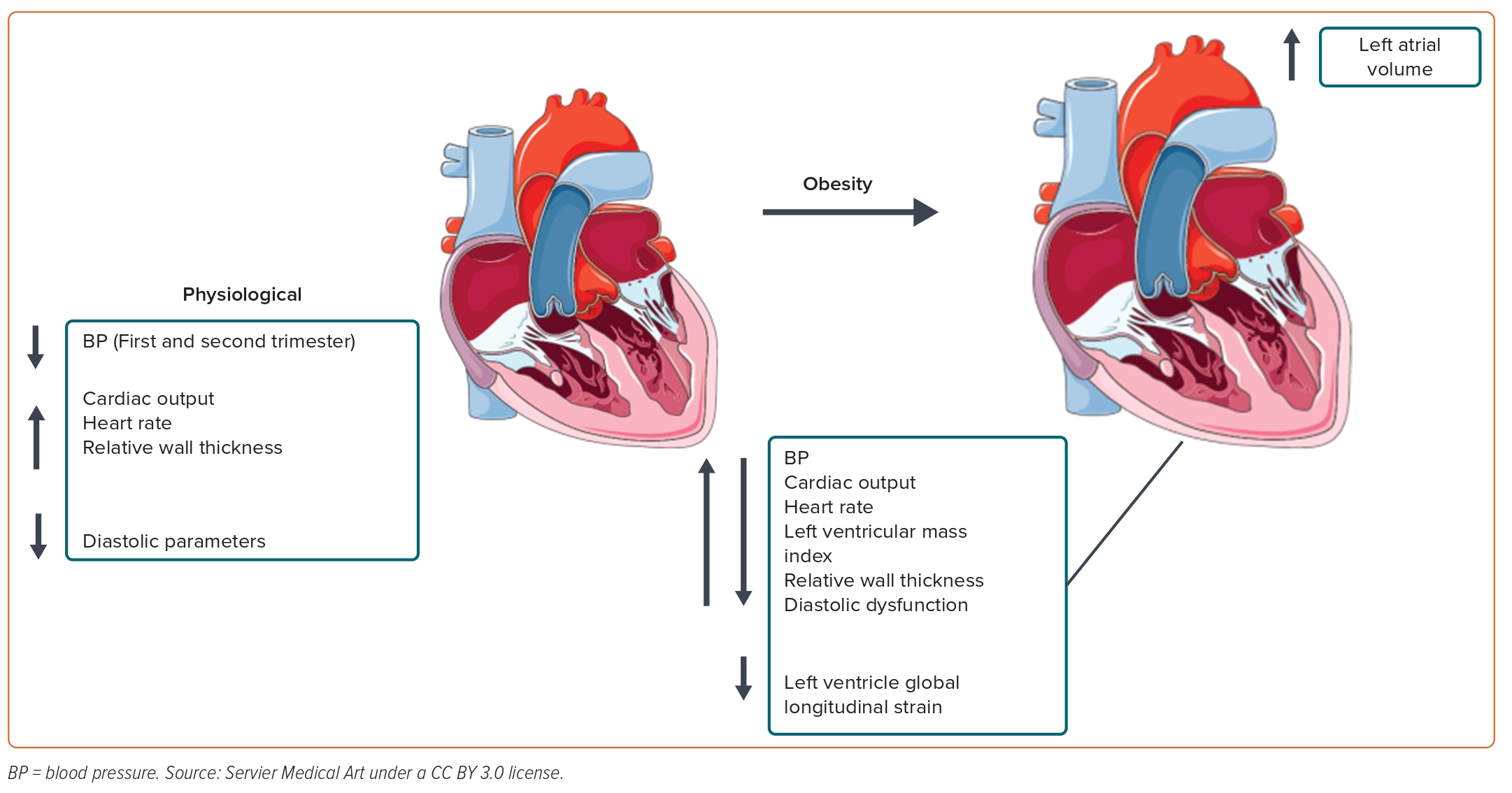
Obesity results in a hyperdynamic circulation with increased blood volume to meet the perfusion demands of the increased adipose tissue. CO is raised due to increased SV and heart rate.33 Obesity is associated with increased stroke workload, leading to left ventricular (LV) dilatation, which, in turn, results in increased wall stress and myocardial mass to compensate.33 Obesity-related hypertrophy is associated with three main changes, namely myocyte hypertrophy, the accumulation of fibrous tissue within the cardiac interstitium and increased epicardial adipose mass, which can lead to alterations in tissue texture and compliance.34,35 These variations can lead to diastolic dysfunction due to altered ventricular filling as a result of abnormal relaxation of the ventricle, increased mass and, therefore, greater reliance on atrial contraction.33,36 Systolic function is usually preserved or even raised in early obesity.36,37 However, conduction and contractility can be compromised when fat deposition occurs in the myocardial tissue and, in severe sustained obesity, systolic dysfunction can ensue.33,38
The mechanism of obesity-related hypertension is complex, but there appears to be an upregulation of the RAAS despite volume expansion.39 Increased angiotensin and aldosterone production may be mediated through several pathways: adipocytes themselves can produce angiotensinogen and renal parenchymal compression, due to visceral fat around the kidney, can lead to decreased renal blood flow, which is sensed by the macula densa and can result in activation of the RAAS.39 Furthermore, there is extra-adrenal aldosterone production via visceral adipocytes that is not affected by traditional RAAS feedback dynamics.39 Along with RAAS upregulation, there is activation of the sympathetic nervous system (SNS), which also plays a role in hypertension by increasing baseline sympathetic tone.39 In recent years, cytokines and adipokines (e.g. leptin) have been thought to play an important role in the development of obesity-related hypertension.39
A limited number of studies have investigated the effect of obesity on the maternal cardiac system. In one study, 40 pregnant women with obesity (BMI ≥35 kg/m2) were found to have higher BP, CO, CO index, LV mass and relative wall thickness (RWT), with a higher incidence of diastolic dysfunction (40% versus 12.5%), at term than healthy (BMI ≤30 kg/m2) pregnant women.40 Furthermore, LV global longitudinal strain, a measure of systolic function, was reduced in the group with obesity (Figure 1).40
In contrast, a study of 23 pregnant women with morbid obesity (BMI ≥40 kg/m2) found that the SV index and CO index were significantly lower and that the systemic vascular resistance index was significantly higher in the morbidly obese group, after correction for body surface area compared to the control group (BMI 20–29.9 kg/m2).41 A longitudinal study in each trimester of pregnancy (n=232) using impedance cardiography, rather than echocardiography, reported a high-volume, low-resistance circulation in pregnant women with obesity (BMI ≥30 kg/m2) compared with pregnant women of normal weight in the first and second trimesters; however, in the third trimester, CO decreased in the group with obesity but not in the normal weight group.42 In addition, there was no longer a difference between the two groups in peripheral vascular resistance (PVR) in the third trimester.42 All these differences between obese and non-obese groups suggest that obesity is associated with a maladaptive cardiac response to pregnancy.
In our own study following pregnant women with obesity (BMI ≥30 kg/m2) or normal BMI longitudinally during pregnancy, we found that women with obesity had higher BP, heart rate and CO and lower PVR than women with a normal BMI. Furthermore, cardiac geometry was altered in the group with obesity, with higher left atrial diameter, LV end-diastolic diameter, intraventricular septal thickness, posterior wall diameter, RWT and LV mass. There was also evidence of impaired diastolic indices in the group with obesity, with a lower E/A ratio, tissue Doppler imaging E′ at the lateral and medial mitral annulus and higher left atrial volume, suggesting suboptimal diastolic function. Finally, women with obesity had reduced longitudinal function, as assessed by mitral plane annular systolic excursion, between the second and third trimesters of pregnancy, indicating possible early cardiac dysfunction in this group, which was not the case in the group with a normal BMI.43
Maternal Cardiac Function and Hypertension
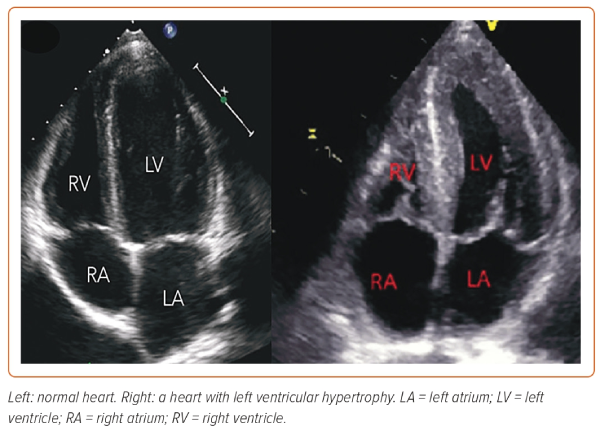
Studies of maternal cardiac function in chronic hypertension are limited. Echocardiographic data (n=130) of women with chronic hypertension and GH shows higher RWT and LV mass index than in normotensive pregnant women.44 Further to this, diastolic functional parameters were reduced in the hypertensive compared with normotensive women, with a lower E/A ratio and higher E/E′. BMI was associated with LV hypertrophy in hypertensive pregnant women (Figure 2).44 In addition, women with chronic hypertension had a higher prevalence of concentric remodelling, in contrast to eccentric remodelling in women with GH and normotensive women.44 A further study of chronic hypertensive women in the third trimester (n=60) found that one-half had subclinical abnormal cardiac function with lower global longitudinal strain.45 Although many women with chronic hypertension do well in pregnancy, they are at increased risk of complications, including superimposed PE, foetal growth restriction, placental abruption and preterm birth.46
In recent years, maternal cardiac function has been associated with the pathophysiology of placenta-related complications, such as PE and/or intra-uterine foetal growth restriction.6,47,48 PE is the leading cause of maternal morbidity and mortality in the developed world, with a prevalence of around 2–5%.49,50 It is thought to derive from abnormal placental development leading to inadequate perfusion and maternal endothelial dysfunction. More recently, studies have linked the development of PE with cardiovascular risk factors, including obesity, age, ethnicity, smoking, chronic kidney disease and diabetes, and it is conceivable that the maternal cardiovascular system plays a vital role in the development of the condition.6 Furthermore, in women who develop PE, the risk of cardiovascular morbidity postpartum as well as in later life is increased.6,51,52
An echocardiographic study of women in the second trimester, prior to the diagnosis of PE, found evidence of LV concentric remodelling, and in those with preterm PE (requiring delivery before 37 weeks gestation) there was a low CO and high resistance with diastolic and systolic dysfunction.53 A large systematic review of cardiac function in women that included studies prior to the onset of GH or PE and studies in those with established GH or PE (n=745 women with GH, n=815 women with PE) reported significant cardiac changes in pregnancies complicated by hypertensive disease.54
Overall, studies in early pregnancy, before the onset of PE, show a hyperdynamic circulation with high CO and low resistance, whereas in the second trimester, women who went on to develop PE had lower CO and higher resistance.54 In the clinical phase of PE, there was a haemodynamic cross-over with reduced CO and high resistance.54 In those with established hypertension, there was concentric LV hypertrophy and diastolic function was impaired, with a reduction in the E/A ratio and an increase in the ratio of peak early mitral inflow velocity (E) over the early diastolic mitral annular velocity (E′). There was no difference in systolic function in most studies, and CO varied.54
Maternal Cardiac Function and Diabetes
In normal pregnancy, compared with the pregravid status, there is a 40–50% increase in insulin resistance that is progressive from mid-pregnancy through to the third trimester; in cases of GDM, this increase in insulin resistance progresses to levels seen in a non-pregnant woman with T2D.55 The physiological rise in insulin resistance is thought to occur in order to provide the foetus with an adequate glucose supply for growth and development.56 It is conceivable that the increased insulin resistance is due to the effect of placental hormones and, to a lesser extent, increased fat deposits during pregnancy. Cortisol and progesterone are most influential, but human placental lactogen, prolactin, oestradiol and other placental hormones such as leptin, tumour necrosis factor-α and resistin are also involved in the decrease in insulin sensitivity occurring during pregnancy.56 In response to this, there is increased production of insulin from pancreatic β-cells to ensure that glucose homeostasis is maintained with minimal effect on glucose levels. Insulin secretion increases as pregnancy progresses, reaching levels twice as high at the end of pregnancy as levels in non-pregnant women.57 GDM is the result of impaired glucose tolerance due to pancreatic β-cell dysfunction on a background of chronic insulin resistance.13
The National Institute of Health and Care Excellence (NICE) guidelines for diagnosing GDM include a fasting plasma glucose concentration ≥5.6 mmol/l and/or a 2-hour plasma glucose level ≥7.8 mmol/l.58 Although the exact pathophysiology of GDM is uncertain, obesity, autoimmunity and genetic reasons have been implicated.58 Obesity predisposes to high blood glucose levels and insulin resistance, tipping women from physiological glucose homeostasis to pathological GDM; insulin resistance prevents glucose from entering the cells, resulting in elevated blood glucose levels. Usually, glucose tolerance returns to normal postpartum in most women with GDM. However, long-term data suggest that 50% of women with GDM will develop T2D within 5 years.58
It has been shown that GDM affects the maternal cardiac system. A cross-sectional study at 26–40 weeks gestation reported that women with GDM (n=123), compared with controls, had lower global longitudinal strain of the left and right ventricles; there was no significant difference between the groups in EF, LV mass and diastolic function.59 Similarly, a smaller study in the second and third trimesters found that women with GDM (n=18) had preserved LV size and function, but LV longitudinal strain was lower than in women with uncomplicated pregnancies, suggesting subclinical myocardial dysfunction.60
Conversely, a study of 40 women with GDM at term reported that these women had a higher BP, heart rate, LV RWT and diastolic indices (E and A), together with lower LV global longitudinal strain, than healthy controls.61 Furthermore, a small study during the third trimester showed that women with GDM (n=21) had different cardiac geometry than women with uncomplicated pregnancies, including higher LV posterior wall and interventricular septal thickness with increased LV mass; there was a positive correlation between LV mass index and fasting glucose.62
Finally, a study in the third trimester and postnatally found that, during pregnancy, women with GDM (n=73), compared with controls, had a higher E/E′ ratio, LV mass indexed for body surface area and lower E/A ratio and global longitudinal systolic strain.63 At 6 months postpartum, the cardiac functional indices improved in both groups, but to a lesser extent in women with GDM.63
It has also been shown that maternal GDM can affect the foetal cardiac system. A study of paired third trimester maternal and foetal cardiac function (n=161) described subclinical cardiac changes in women with GDM, including lower LV diastolic and systolic (tissue Doppler systolic [s′] wave) functional indices, compared with women with uncomplicated pregnancies.64 In addition, foetuses of GDM mothers had more globular-shaped hearts with increased right ventricle and LV sphericity indices and reduced systolic functional indices, compared with foetuses of women who were normoglycaemic. The effect of GDM on maternal and foetal hearts was different, and there was no clear association between the two.64
The impact of GDM is not just confined to pregnancy. A systematic review and meta-analysis found that women with GDM have a twofold higher risk of cardiovascular events in the first decade after the index pregnancy compared with controls.65 The risk did not depend on the development of T2D and showed that women with GDM are at risk of future cardiovascular disease, which presents an opportunity for early risk factor modification.65
At the time this review was written, there were no studies investigating the maternal cardiac function in women with pre-existing T1D or T2D.
Maternal Cardiac Function and Metabolic Syndrome
Obesity is a component of metabolic syndrome that can be diagnosed outside of pregnancy if three of the following five criteria are met: increased waist circumference, hypertriglyceridaemia, low HDL, high BP and high fasting glucose.66,67 The underlying cause of metabolic syndrome is obesity, lack of physical activity and genetic predisposition. Excess adipose tissue and tissue dysfunction lead to insulin resistance. Proinflammatory cytokines, such as tumour necrosis factor, leptin, adiponectin, plasminogen activator inhibitor and resistin, are released from the enlarged adipose tissue and result in inappropriate insulin handling.66,67 Impairment of the signalling pathway, together with insulin receptor defects and defective insulin secretion, leads to insulin resistance. Eventually, there is vascular and autonomic damage resulting in metabolic syndrome.66,67
A large prospective study of women with metabolic syndrome in early pregnancy found that they were at an increased risk of PE and GDM by a factor of 1.63 and 3.71, respectively.21 There are no human studies assessing the maternal cardiac function in women with prepregnancy metabolic syndrome. There is only one animal study of pregnant female mice, where metabolic syndrome was induced by feeding a Western diet but not in pregnant mice fed a control diet.68 The former group had increased cardiac mass and showed signs of pathological cardiac hypertrophy, including fibrosis and upregulation of foetal genes associated with pathological hypertrophy. In addition, postpartum, mice had signs of cardiac dysfunction when challenged with angiotensin II and phenylephrine infusion.68
Bariatric Surgery
Weight loss achieved by diet and physical exercise may improve the cardiovascular risk profile, but in most patients, lifestyle changes and pharmacological interventions do not achieve long-term weight loss.69–71 Bariatric surgery is a successful treatment for sustainable weight loss and achieves around 55% excess body weight loss across different surgical methods.72,73 Studies on the long-term follow-up of patients after bariatric surgery have shown improvement or resolution of hypertension and T2D, with a 40% reduction in any-cause mortality.39,74 The NICE guidelines on obesity recommend bariatric surgery for a BMI ≥40 kg/m2, or a BMI of 35–40 kg/m2 with comorbidities such as diabetes or hypertension.75 There are several different types of bariatric surgery; the adjustable gastric band is a minimally invasive laparoscopic procedure where a ring with an inner inflatable band is placed around the fundus of the stomach to create a small pouch.76–78 In sleeve gastrectomy, the stomach is reduced to approximately 15% of its original size, and the procedure can achieve an approximate 60% reduction of excess body weight, comparable to gastric bypass.76–78 Roux-en-Y gastric bypass (RYGB) first involves the creation of a small, 15 to 30 ml pouch from the upper stomach with bypass of the remaining stomach, which restricts the volume of food. The second part of RYGB involves dividing the proximal small intestine, with the lower portion anastomosed to the gastric pouch and the upper allowing transfer of gastric acid and enzymes from the bypassed stomach. The procedure achieves around 60% excess weight loss.76–78 Biliopancreatic diversion with duodenal switch is a less common procedure and the surgery achieves the highest amount of excess weight loss, approximately 70%.76–78
Bariatric Surgery and Cardiac Function
The harmful effect of obesity may be due to its association with an increase in inflammatory processes and enlargement of adipocytes.79 Adipocytes recruit macrophages, which are responsible for much of the cytokine production in adipose tissue and promote inflammation, as well as the release of several factors that influence insulin resistance and other associated complications, such as hypertension, atherosclerosis, non-alcoholic fatty liver disease, T2D, cancer and death.79–81
Many studies have found a reduction or resolution of hypertension after bariatric surgery due to a combined effect of weight loss and changes in gastrointestinal peptides.39 It has been reported that BP is lowered as early as 1 week after surgery and before any meaningful weight loss has occurred, suggesting an effect from the surgery itself.82,83 It is thought this is due to an early reduction in SNS activity, because decreased sympathetic activity and improvement in baroreceptor modulation of heart rate and sympathetic activity have been shown after RYGB.84,85 It has been suggested that baroreflex impairment is a possible cause of the obesity-related sympathetic activation.84,86 An improvement in insulin sensitivity and a reduction in leptin after bariatric surgery may also be involved, because both lead to direct stimulation of the SNS and may be regulators of arterial pressure, independent of BMI or body fat percentage.39,87 Increased plasma concentrations of glucagon-like peptide-1 (GLP-1) immediately after RYGB are also known to improve insulin resistance and may have a natriuretic and diuretic effect.82,88–90
Bariatric surgery results in beneficial effects on cardiac structure and function. A large meta-analysis reviewed individuals (n=1,486) before and after surgery, with follow-up ranging from 3 to 45 months.91 Thirty-seven studies used echocardiography data and five used cardiac MRI. Favourable changes in cardiac geometry included reductions in LV mass, LV mass index and LV end-diastolic and end-systolic volumes.91 There was a small improvement in systolic function, assessed by EF before and after bariatric surgery (weighted mean increase of 1.198%).91 However, studies using strain reported greater improvements in systolic function.92,93 Diastolic function assessed by the E/A ratio and left atrial indices also improved after bariatric surgery.91 Traditionally, it was thought that cardiac remodelling after bariatric surgery was due solely to haemodynamic changes, such as lowering of BP, and weight loss, but it is now accepted that the metabolic components of bariatric surgery that contribute to cardiac reverse remodelling include the systemic BRAVE (Bile flow alteration, Reduction in gastric size, Anatomical gut rearrangement, Vagal manipulation and Enteric gut hormone manipulation) effects.91 Manipulation of the enteric gut hormones has been shown to have beneficial effects on cardiac function via the entero–cardiac axis.91,94,95 Hormones such as secretin, glucagon and vasoactive intestinal peptide act as inotropes by activating cardiac membrane adenylate cyclase, a key enzyme in cardiac cell communication. Moreover, other key hormones that are altered as a result of bariatric surgery, including GLP-1, leptin, adiponectin and ghrelin, are also known to modulate cardiac function.91,94–96 Although there is a large body of evidence of cardiovascular benefits of bariatric surgery, it has been reported that 1 year after surgery in patients with severe obesity, LV longitudinal function was largely recovered; however, LV mid-wall mechanics did not improve, particularly in women and patients with persistent LV geometric abnormalities.97
Although bariatric surgery is the most successful method of weight loss, weight regain is a common phenomenon, with around 20–25% of patients struggling with clinically significant weight regain.98 This can be ameliorated with a multidisciplinary approach consisting of behavioural modification, psychological counselling (which has been shown to improve physical and mental wellbeing after bariatric surgery), and secondary interventions, such as the use of an appetite suppressant and endoscopic plication or revision surgery.99,100 Adjunct therapy with semaglutide has been shown to have a clear benefit in post-bariatric surgery weight regain.101
Pregnancy After Bariatric Surgery
Bariatric surgery has several maternal and neonatal implications. It is advised that pregnancy is avoided for around 18–24 months after bariatric surgery to allow for maximum weight loss and to achieve weight stabilisation, because rapid weight loss and potential micronutritional deficiencies may have adverse outcomes on the offspring. During pregnancy, regular reviews and dietician input, along with micronutrient supplements, are required to avoid deficiencies.102
Studies have shown that in women with previous bariatric surgery, there is a lower incidence of GDM, PE and LGA neonates, but a higher incidence of SGA neonates and preterm birth.24 A large meta-analysis reported on studies that compared pregnant women after bariatric surgery to women matched for pre-surgery BMI and women matched for pre-pregnancy BMI.24 Post-bariatric pregnant women had a lower incidence of GDM and PE, by approximately 50%, compared with women matched for pre-surgery BMI, but there was no difference compared with pregnant women matched for pre-pregnancy BMI.24
In another review, the incidence of PE was reported to be lower in pregnancies of obese women after bariatric surgery than in obese women without surgery, with the authors concluding that surgery improves outcomes even when obesity is still present; however, rates of PE were still higher than in women of normal weight.103 Further to this, other studies have found lower rates of hypertensive disease in post-bariatric pregnant women than in women with similar pre-pregnancy BMI, indicating that the effect of the surgery on the development of hypertensive disease is independent of the weight loss.104,105 Overall, bariatric surgery has been associated with lower rates of maternal complications (hypertensive disorders and GDM), and the rates of these complications may approach community rates.106,107
Although there are maternal benefits of bariatric surgery, a large systematic review and meta-analysis reported that previous bariatric surgery in women is associated with increased odds of perinatal mortality, congenital anomalies, preterm birth, SGA neonates and neonatal unit admission.108 Malabsorptive procedures (RYGB and biliopancreatic diversion with duodenal switch) were associated with a significant increase in SGA neonates and a decrease in LGA neonates, whereas restrictive procedures (sleeve gastrectomy and gastric band) were not.108 It is likely that the increase in adverse perinatal outcomes is related to maternal malnutrition.108 When further comparing the operations, pregnant women with previous gastric band and sleee gastrectomy had a lower risk of anaemia and GDM than those with gastric bypass.109 Infants born to mothers after gastric banding had a higher birthweight than those born to mothers after gastric bypass, but the risk of preterm birth was higher in women with gastric banding than in those with gastric bypass.109
Maternal Cardiac Function Following Pre-pregnancy Bariatric Surgery
Studies on the maternal cardiovascular system in pregnancies following bariatric surgery are sparse. We have previously reported that women with a history of bariatric surgery have a better cardiac adaptation to pregnancy than women with a similar early-pregnancy BMI.110 We studied women (n=41 in each group) longitudinally (in the three trimesters) during pregnancy and found that, compared with the no-surgery group, women with previous bariatric surgery had lower BP, heart rate and CO, with evidence of more favourable diastolic indices, including a higher E/A ratio, higher TDI E′ and a lower left atrial volume. Furthermore, women with previous bariatric surgery had lower global longitudinal and circumferential strain, suggestive of better systolic function.110 When post-bariatric pregnant women were compared, longitudinally, to pregnant women without surgery but with early pregnancy BMI similar to the pre-surgery BMI of the post-bariatric women (n=30 in each group), we found that the former group had more favourable haemodynamic indices, diastolic and systolic function and altered cardiac geometry with lower LV mass and RWT.111 It is conceivable that the cardiac changes observed in pregnant women with previous bariatric surgery may contribute to the lower prevalence of hypertensive disorders seen in this population.
Management of High-risk Women with Metabolic Disorders in Pregnancy
The management of women with chronic hypertension, diabetes or metabolic syndrome requires the input of a specialist obstetric team. Women with chronic hypertension taking an angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker should be changed to labetalol or nifedipine to avoid foetal risks, with a target BP of 135/85 mmHg. Renal function and the protein:creatinine ratio should be checked. Women with chronic hypertension are at high risk of developing superimposed PE, and aspirin prophylaxis is advised from 12 weeks to reduce this risk.112 Women with pre-existing diabetes should have preconception care to plan their pregnancy because good blood glucose control before conception and throughout their pregnancy will reduce the risk of miscarriage, congenital malformation and stillbirth. Metformin and insulin are safe to continue in pregnancy, but it is advised that statins are stopped.58 Retinal assessment, renal function and the protein:creatinine ratio should be assessed in women with pre-existing diabetes. All pregnant women with risk factors for developing GDM (BMI >30 kg/m2, previous macrosomic baby, previous GDM, family history of diabetes, an ethnicity with a high prevalence of diabetes) are tested during pregnancy with an oral glucose tolerance test. Gestational diabetes is diagnosed if there is a fasting plasma glucose level of ≥5.6 mmol/l or a 2-h plasma glucose level of ≥7.8 mmol/l.58 Management includes a trial of diet and exercise changes, metformin and insulin.58
In recent years, GLP-1 receptor agonists have emerged as an important group of drugs used in the treatment of T2D and obesity. GLP-1 receptor agonists are contraindicated in pregnancy and breastfeeding because animal studies have reported foetal defects.113 Women on these medications have improvements in weight loss, menstrual regularity and therefore fertility, which raises the need for effective contraception and pregnancy planning.113
Effects on Offspring
All maternal metabolic disorders appear to have a transgenerational effect. Foetuses of mothers with obesity are at increased risk of complications such as congenital anomalies, stillbirth, prematurity, macrosomia and neonatal death.114 In childhood, offspring of mothers with obesity are at increased risk of developing obesity; in adulthood, maternal obesity has an impact on cardiometabolic health, with dysregulation of metabolism, including glucose/insulin homoeostasis and the development of hypertension and vascular dysfunction, which is thought to be related to ‘foetal programming’.4,115,116 With regard to cardiac implications, a systematic review of maternal obesity and offspring cardiac function reported that, compared with offspring of women without obesity, the foetuses of women with obesity had lower LV strain that persisted in infancy.117 In addition, infants of mothers with obesity appear to have a thicker interventricular septum and worse diastolic indices compared with foetuses of non-obese mothers.118
GDM and pre-existing diabetes can also impact foetal heart organogenesis and the feto–placental circulation. The severity of the foetal cardiac ‘damage’ relates to the type of diabetes, HbA1c level in early pregnancy and the degree and duration of hyperglycaemia and hyperketonaemia, which are both toxic to the developing embryo.119 Foetal and neonatal effects of GDM and pre-existing diabetes include congenital cardiac malformations, foetal cardiomyopathy, foetal venous thrombosis and pathological foetal heart rates.119,120 A review on maternal GDM and pre-existing diabetes (n=1,925; 39 studies) reported that foetal cardiac hypertrophy and impaired diastolic function were observed in most studies.121 The association between maternal diabetes and foetal systolic function was inconsistent; however, recent studies using strain reported decreased systolic function in these pregnancies.121
Most studies assessing the overall foetal cardiac function found significant impairment in diabetic pregnancies, with increased intraventricular septal thickness and a decreased E/A ratio, and similar results for GDM and pre-existing diabetes pregnancies.121 In the long term, the offspring of diabetic mothers have increased rates of early-onset cardiovascular disease, from childhood to early adulthood, reported to be 29% greater than for children from non-diabetic mothers.122
Conclusion
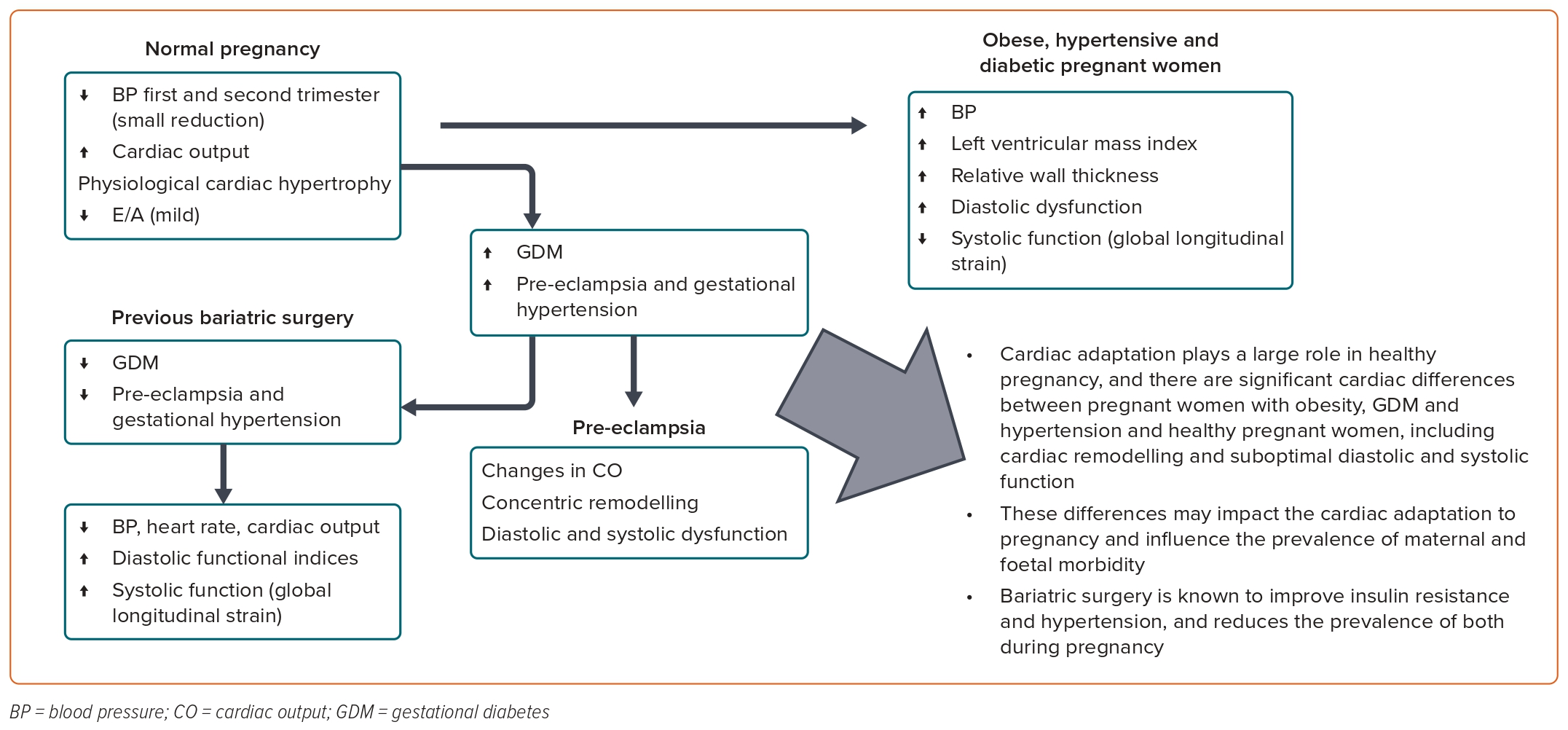
Obesity and its associated consequences of hyperglycaemia, hypertension and metabolic syndrome can significantly impact pregnancy and the maternal cardiac system (Figure 3). Cardiac adaptation plays a large role in a healthy pregnancy; women with obesity and/or diabetes exhibit significant cardiac differences compared with healthy pregnant women, including LV hypertrophy and suboptimal diastolic and systolic function. These differences may impact the cardiac adaptation to pregnancy and influence the prevalence of maternal and foetal morbidity. Bariatric surgery is known to improve insulin resistance and hypertension and reduces the prevalence of both during pregnancy. As the obesity epidemic grows, metabolic disorders in pregnancy are of increasing importance, and pregnancy provides the opportunity to identify modifiable risk factors to improve longer-term cardiovascular health in women, as well as in their offspring.