Chronic kidney disease (CKD) is increasingly prevalent in patients with heart failure (HF) and HF is one of the leading causes of hospitalisation, morbidity and mortality in patients with impaired renal function.1
There is a significant co-occurrence of HF and CKD – it is reported that almost half of patients with HF have a degree of renal impairment and HF is prevalent in 17–50% of patients with CKD, depending on the stage of the CKD and age of the patients.2,3 In addition, the prevalence and mortality of HF increases with worsening renal failure.1 Renal function is an independent predictor for inpatient mortality of patients with acute HF, length of hospital stay and re-admission rate.1
With improving survival in both patients with HF and those with CKD, it is likely that the numbers of patients presenting with both these conditions will continue to rise.
Pathophysiology and the Interdependence of Heart and Kidney Function
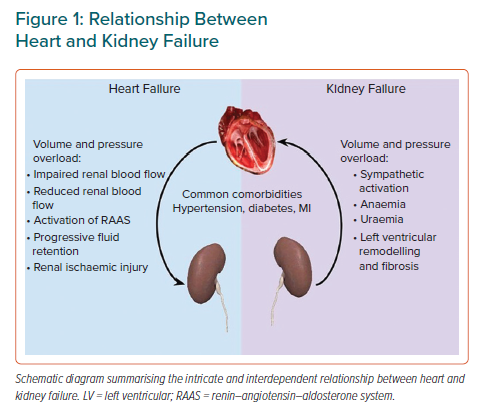
The overlap between HF and CKD can be explained by their similar aetiological factors, including hypertension and diabetes as well as intertwined physiological processes.1 HF precipitates and perpetuates CKD via a reduction in renal blood flow, impaired renal haemodynamics and resultant ischaemic injury.4 Conversely, CKD contributes to progressive left ventricular (LV) remodelling, fibrosis and cardiac dysfunction as a result of fluid overload, anaemia, uraemia, excessive renin–angiotensin–aldosterone and sympathetic activation among other factors (Figure 1).1,4 Therefore, it is not surprising that HF and CKD have synergistic effects, with the presence of one speeding up the process of the other.
Heart Failure and Chronic Kidney Disease
Heart Failure Management in Patients with Chronic Kidney Disease
While CKD accounts for a growing burden of morbidity and mortality in patients with HF, the management of this condition remains difficult. Generally, there is less use of HF medication as patients with HF and CKD may be more susceptible to the renal and metabolic effects of various HF therapies.5
Management of combined HF and CKD is more costly and frequently disjointed, with patients often referred back and forth between nephrologists and cardiologists with changes in renal function associated with renin–angiotensin–aldosterone inhibitors (RAASis).6 This results in multiple hospital attendances and often discontinuation of guideline-directed medical therapy.
To address this, multidisciplinary cardiology and renal clinics have been recommended as an evidence-based tool to significantly reduce all-cause mortality and have also shown trends in reduction all-cause and cardiovascular hospitalisations in patients with CKD.7
Finally, there is a paucity of evidence for the use of HF medications in patients with advanced-stage CKD (stage 4–5).1
Heart Failure Subtypes and Chronic Kidney Disease
This review focuses on HF with reduced ejection fraction (HFrEF), defined as an ejection fraction <40%. However, appreciation for the significant mortality and morbidity of HF with preserved ejection fraction (HFpEF) is growing.
HFpEF refers to the presence of HF symptoms with an ejection fraction (EF) ≥50% and is related to impaired LV relaxation and increased LV stiffness.1,8 The pathophysiology of HFpEF is still being elucidated but several factors have been implicated in the generation of impaired LV filling, including systemic inflammation, LV hypertrophy and endothelial dysfunction.8
HFpEF is now believed to be more prevalent in patients with CKD than HFrEF and poor renal function is likely mechanistically to be involved in HFpEF.8 Despite being the prominent HF subtype, there are few evidenced-based treatments with associated prognostic benefits. The recently published EMPEROR-Preserved study showed an improvement in combined risk of cardiovascular death or hospitalisation for patients with HFpEF treated with empagliflozin, regardless of diabetes status.9
Regarding HF with mid-range ejection fraction, it is currently an evidence-free zone. However, since the introduction of this subtype in the European Society of Cardiology (ESC) guidelines in 2016, there are trials being conducted to focus on this group of patients.
The aim of this narrative review is to synthesise current evidence for treatment of HF in the context of coexisting CKD, with particular emphasis on newer pharmacological and non-pharmacological treatments.
Diuretics
Diuretics play a ubiquitous role in the alleviation of HF symptoms but have no established effect on disease progression or mortality.1 Diuretic therapy is challenging in CKD patients because of the need for higher doses, frequently causing transient worsening renal function, electrolyte imbalances such as hyponatraemia and hypokalaemia.10
The effects of diuretic therapy on mortality and morbidity have not been studied in randomised controlled trials (RCTs). However, a Cochrane meta-analysis has shown that in patients with chronic HF, loop and thiazide diuretics appear to reduce the risk of death and worsening HF compared with placebo and, compared with an active control (existing HF medication), diuretics appear to improve exercise capacity.11
The exact effect of diuretic therapy on trajectory of HF remains unclear, particularly in patients with CKD, who may have an element of diuretic resistance. With regards to HFpEF, there is evidence that diuretics provide symptomatic relief across all EF ranges, although it is unclear whether this finding extends to patients with impaired renal function.
β-blockade
β-blockers have a well-established symptomatic and prognostic benefit in patients with HFrEF.12 They represent one of the four pillars of HF management which also include angiotensin receptor-neprilysin inhibitors (ARNIs), mineralocorticoid receptor antagonists (MRAs) and sodium–glucose co-transporter 2 inhibitors (SGLT2is).8
The beneficial effects of β-blockade in HF are mediated by attenuating the damaging effects of sympathetic activation on cardiac muscle.1 RCTs and subsequent meta-analyses have shown improvements in HF symptoms, LV function and reduction in hospitalisations and mortality with beta-blockade in patients with HFrEF.13–16
Subgroup and post-hoc analysis of large RCTs including MERIT-HF and CIBIS-II support the use of β-blockers in patients with both HFrEF and CKD 1–3.17,18 A recent meta-analysis of 10 double-blinded, placebo-controlled RCTs demonstrated that β-blockers were beneficial in patients with HFrEF and moderate CKD (estimated glomerular filtration rate [eGFR]: 30–60 ml/min/1.73 m2).19 However, there was an insufficient number of patients with advanced CKD to draw any conclusions.19
With regards to specific β-blockers, there is evidence to support the use of carvedilol in patients with advanced CKD. In a cohort of patients with HFrEF on haemodialysis, carvedilol was associated with improved mortality and reduced risk of sudden death.1,20
Despite the established evidence base in HFrEF, there are mixed findings on the utility of β-blockade in patients with HFpEF, with little data available for patients with advanced CKD.1,19
Renin–Angiotensin–Aldosterone Inhibition
Large, multicentre, placebo-controlled RCTs, including SAVE, CONSENSUS and SOLVD (for angiotensin-converting enzyme inhibitor [ACEi]) and CHARM and VALHEFT (for angiotensin II receptor blocker [ARB]), have established the benefits of RAASis in patients with HFrEF with CKD stages 1–3.21–25 Patients with advanced CKD have systematically been excluded from most of these studies.
ACEis have side-effects including hyperkalaemia and rising creatinine, which can be a barrier to their use in patients with HF and CKD.5 Recent observational data show that the prescription of RAASis reduces in a step-wise manner with worsening stage of CKD.5 The changes in renal function associated with RAASis are caused by on-target, efferent arteriolar vasodilation and subsequent decrease in filtration pressure at each nephron. An increase of up to 30% in serum creatinine can be viewed as a direct haemodynamic consequence of RAASis and is generally considered to be benign, with no long-term negative effects.26
In terms of treatment in patients with CKD and HFpEF, ACEis or ARB had no significant effect of on cardiac and all-cause mortality.27 There are also signs from meta-analysis of several trials that worsening renal function associated with ACEi/ARB use may not be as benign in patients with HFpEF as in those with HFrEF.1,27
Mineralocorticoid Receptor Antagonists
There is evidence for the beneficial effects of mineralocorticoid receptor antagonists (MRAs) on hospitalisations and mortality for patients with HFrEF and CKD stage 1–3.28–30
In the RALES study, approximately half of the 1,658 patients included had an eGFR <60 ml/min/1.73 m2 and the risk reduction for hospitalisations and mortality was similar for subjects regardless of kidney function.28 Hyperkalaemia occurred more often in patients with impaired kidney function.28
Furthermore, in patients with post-MI HF (EF <40%), eplerenone has shown benefits in outcomes including death from cardiovascular events or hospitalisation for a cardiovascular event.29 While this study excluded patients with advanced CKD, there was an increased risk of hyperkalaemia in the eplerenone group, but no associated increase in mortality.29
Evidence around the use of any MRA in patients with HF and CKD stage 4–5 is lacking.
For patients with HFpEF, studies such as the Top Cat and I-PRESERVE trial of MRA in general populations with HFpEF failed to show an improvement in their primary outcomes, which included death or hospitalisation with a cardiovascular cause.31,32 These trials excluded patients with advanced CKD (defined in the TOPCAT trial as eGFR <30 ml/min/1.73 m2 and in I-PRESERVE as serum creatinine >221 μmol/l). Similar to ACEi/ARBs in HFpEF, worsening renal function with MRAs was associated with worse outcome in the I-PRESERVE trial.
Sodium–Glucose Co-transporter 2 Inhibitors
SGLT2i therapy in patients with HFrEF has been shown to reduce mortality and hospitalisations independent of diabetic status. It is now one of the four pillars of guideline-directed medical therapy for HF patients.8
The DAPA-HF study compared dapagliflozin to standard care/placebo in 4,744 patients with New York Heart Association (NYHA) class IIIV HF and EF <40%.33 This study included 1,926 (40.6%) patients with CKD stage 3, but excluded those with eGFR <30 ml/min/1.73 m2 or rapidly declining renal function. There was a significant reduction in the primary composite outcome (worsening HF or cardiovascular death: HR 0.74; 95% CI [0.65–0.85]). The reduction in the primary endpoint was similarly observed in CKD and non-CKD patients: HR 0.72 (95% CI [0.59–0.86]) and 0.76 (95% CI [0.63–0.92]).33 There was also evidence for the renal safety of dapagliflozin, with lower rates of renal complications seen in the dapagliflozin group than in those receiving placebo/standard care (composite consisted of 50% sustained decline eGFR, end-stage renal disease or renal death).33
Recently, the EMPEROR-Reduced trial studied the use of empagliflozin in patients with HF, with the primary outcome being cardiovascular death or hospitalisation for worsening HF. Significantly, this trial included patients with an eGFR as low as 20 ml/min/1.73 m2 with 48% of patients having an eGFR <60 ml/min/1.73 m2. Cardiovascular death and HF hospitalisations were reduced by 25% (HR 0.75; 95% CI [0.65–0.86]). The eGFR decline was slower with empagliflozin than with placebo (−0.55 versus −2.28 ml/min/1.73 m2/year), for a between-group difference of 1.7 ml/min/1.73 m2/year (95% CI [1.10–2.37]). There was a 50% (95% CI [32–77]) reduction in renal composite outcome (incidence of renal replacement therapy or sustained loss of eGFR) in patients randomised to receive empagliflozin. Furthermore, empagliflozin was associated with reduced HF hospitalisation, slower eGFR decline and reduced adverse kidney events (a composite of chronic dialysis, kidney transplant or sustained reduction in eGFR).34
Similar to ACEi therapy, initiation of SGLT2i can be associated with an initial benign decline in kidney function. However, there is a net reduction in kidney disease progression for patients both with and without HF.35,36
For example, in the DAPA-HF trial, there was a higher initial decline in eGFR in the dapagliflozin group than in the placebo group (−3.97 ± 0.15 versus −0.82 ± 0.15 ml/min/1.73 m2). However, thereafter, the annual change in the mean eGFR was smaller with dapagliflozin than with placebo (−1.67 ± 0.11 and −3.59 ± 0.11 ml/min/1.73 m2, respectively), for a between-group difference of 1.92 ml/min/1.73 m2/year (95% CI [1.61–2.24]).33
Angiotensin Receptor and Neprilysin Inhibitors
Neprilysin is a naturally occurring endopeptidase that is responsible for the degradation of various vasoactive peptides, including natriuretic peptides.37 Neprilysin inhibitors potentiate the effect of the natriuretic peptide system, a key counter-regulatory system against excessive RAAS activation.37
Clinically, neprilysin inhibitors are combined with angiotensin-receptor blockers such as sacubitril/valsartan. The recently published 2021 ESC HF guidelines suggested that either ACEis or ARNIs are a core component of the triad of therapeutic agents in HF, alongside β-blockade and MRAs.8 ARB monotherapy is then used for patients who are intolerant of either ACEis or ARNIs.8 Current guidelines advocate for the substitution of ACEi/ARB with an ARNI in patients who still have symptoms despite optimal medical therapy and who have an eGFR >30 ml/min/1.73 m2.8 ARNI therapy is now is recognised as one of the four pillars of guideline-directed medical therapy for HF.8
The importance of ARNIs was established in the PARADIGM-HF study, which showed the superiority of ARNI over enalapril for patients with HFrEF.38 The primary outcome of this study was a composite of either cardiovascular mortality or hospitalisation for HF, and the trial was stopped early as ARNIs had clear benefits over ACEis (HR 0.8; 95% CI [0.73–0.87]). With regards to patients with CKD, this study excluded patients with eGFR <30 ml/min/1.73 m2. There was evidence that sacubitril/valsartan had less of a nephrotoxic effect than enalapril, with fewer participants stopping sacubitril/valsartan because of renal impairment than enalapril (0.7 versus 1.4%; p=0.002). The rate of decline in eGFR was slower with ARNIs than with ACEis or ARBs.38 Side-effects such as hyperkalaemia were less common than with ACEis or ARBs.
A meta-analysis of all trials suggested a lower incidence of serious hyperkalaemia (defined as K >6.0 mmol/l) with ARNIs compared to enalapril or valsartan with a pooled relative risk of 0.76 (95% CI [0.65–0.89]) and a lower incidence of worsening kidney function relative risk (RR) of 0.79 (95% CI [0.67–0.95]).39 A more recent RCT included patients with an eGFR as low as 20 ml/min/1.73 m2 and demonstrated safety and efficacy similar to irbesartan.40
In patients with HFpEF, the PARAGON-HF trial compared the effect of sacubitril/valsartan with valsartan monotherapy on hospitalisations and death from cardiovascular causes.41 Of note, this trial excluded patients with an eGFR <30 ml/min/1.73 m2. The trial failed to meet the primary endpoint, but did show a non-significant reduction in HF hospitalisations and improved quality of life. There was evidence of heterogeneity in clinical response across specified subgroups, with better outcomes seen in women and patients with lower EFs who were randomised to receive sacubitril/valsartan. There was no significant interaction found with renal function, although patients with CKD at stage 4 or greater were excluded.
Ivabradine
Raised resting heart rate has been found to be a risk factor for mortality and cardiovascular outcomes in observational studies of patients with HF.42
Ivabradine is a selective inhibitor of the sino-atrial I(f) current inhibitor that has an isolated negative chronotropic effect, with negligible effects on cardiac contractility and conductivity. The SHIFT-HF study demonstrated an improvement in cardiac death and HF hospitalisations when used in patients with stable HFrEF on established β-blocker therapy (HR 0.82; 95% CI [0.75–0.90]).42 The study included patients with creatinine levels <220 µmol/l. Results were similar in patients with and without renal dysfunction and ivabradine had no significant effect of on kidney function. However, RCT evidence is lacking to support the safely and efficacy of ivabradine in patients with CKD stage 4–5.
There are only small-scale studies assessing the impact of ivabradine on patients with HFpEF. Cacciapuoti et al. studied the effect of ivabradine on echocardiographic markers of LV diastolic dysfunction.43 At 3 months, a significant improvement in LV diastolic function was noted (echocardiogram markers include diastolic mitral inflow E/A ratio, deceleration time of early diastolic flow mitral valve and S/D wave ratio). Other studies failed to show an improvement in other echocardiographic markers (echo-Doppler E/e’ ratio), 6-minute walking test or plasma NT-proBNP.44 The utility of ivabradine in the context of HFpEF with renal impairment is unknown.
IV Iron Therapy
Iron deficiency is common in patients with HF and CKD and is associated with worse functional outcomes, morbidity and mortality.1 In patients with CKD, iron deficiency is likely to be multifactorial in origin with bone marrow dysfunction, chronic inflammation and dietary deficiencies among other factors implicated in the pathogenesis.1,45
From a clinical perspective, it is important to be cognisant of other causes of iron deficiency and ensure serious causes such as gastrointestinal malignancy are ruled out. The risk associated with iron deficiency is independent of anaemia and is a stronger risk factor for adverse outcomes compared to anaemia status.45 In line with this, IV iron therapy has shown benefits in quality of life, functional outcomes and risk of hospitalisation in patients with iron deficiency and HFrEF while erythropoiesis-stimulating agents (ESA) have no known effect on HF outcomes and are associated with increased stroke risk.45–48
The indications for starting iron therapy in patients with CKD are different from those for patients with HF.
Patients can qualify for IV iron therapy based on meeting either HF or CKD criteria. In HF, it is recommended to commence IV iron in symptomatic patients (NYHA class 2–4) with EF <50% and iron deficiency (defined as either serum transferrin <100 μg/l or serum transferrin 100–299 μg/l with transferrin saturation <20%) regardless of anaemia status.
For patients with CKD, Kidney Disease Improving Global Outcomes (KDIGO) 2012 guidelines recommend a trial of IV iron therapy in patients with anaemia who are not on ESA therapy if an increase in haemoglobin is desired and transferrin saturation is <30% and ferritin <500 μg/l.
It is vital that patients receiving ESA therapy are iron replete to account for the anticipated increase in erythropoiesis. A trial of IV iron therapy is recommended in these patients to increase haemoglobin concentration, facilitate a reduction in ESA dose and biochemical evidence of iron deficiency (transferrin saturation <30% and ferritin <500 μg/l).
In CKD patients without LV systolic dysfunction (LVSD), decisions regarding administration route are informed by patient preference, options for administration of IV iron during dialysis sessions, tolerance of side-effects (namely gastrointestinal side-effects with oral preparations), risk of anaphylactoid reactions with IV preparations and patient compliance.
The PIVOTAL trial has established the benefits of a high-dose, proactive IV iron regimen, compared to low-dose, reactive regimen in patients on haemodialysis (HD). The benefits of this regimen include fewer HF hospitalisations and combined cardiovascular endpoints, without excess infections.49 Current available evidence suggest that, in patients with an EF <50%, only IV iron is beneficial.45–48 In practice, patients receiving dialysis will often have IV iron following a dialysis session. There are ongoing studies to establish the effects of oral iron in this population.
The benefits of IV iron (in patients with EF <50%) in improving quality of life, relieving symptoms of HF and reducing the risk of hospitalisation have been established by several RCTs, including FAIR-HF, CONFIRM-HF, EFFECT-HF and AFFIRM-HF.45–48
The primary outcome of the majority of these studies was improvement in HF symptoms; only one study (AFFIRM-HF) was specifically designed to detect a difference in HF hospitalisation and mortality.45 This study showed a reduction in HF hospitalisations with no effect on mortality.45
A meta-analysis showed that hospitalisations for HF and mortality were significantly decreased in the iron-treated group, of whom >40% had an eGFR <60 ml/min/1.73 m2 and iron deficiency (ferritin levels <100 μg/l or <300 μg/l if transferrin saturation was <20%) irrespective of haemoglobin level.50
However, a more recent meta-analysis of these four studies (n=2,042) confirmed that IV iron therapy was associated with a reduction in HF hospitalisations (pooled RR 0.69; 95% CI [0.61–0.78]) with no statistically significant impact on cardiovascular or all-cause mortality.51 Of note, many of these studies, including AFFIRM-HF, did not specifically exclude patients with CKD. In total, 40% of patients included in AFFIRM-HF had CKD stage 3 or higher and the response was similar in patients with and without CKD.45
There are no studies on IV therapy in patients with HD and HFpEF. The FAIR-HFpEF study will provide clarification on the utility of IV iron in patients with HFpEF (NCT03074591).
Novel Pharmacological Agents
Novel agents, such as soluble guanylate cyclase stimulators (e.g. vericiguat), combined hydralazine and isosorbide dinitrate and potassium binders have been proposed as adjuvant HF therapies.
Vericiguat has subsequently been incorporated into the 2021 ESC HF recommendations for patients who have worsening symptoms despite ACEi/ARNI, β-blockade and an MRA.8 Studies involving vericiguat included patients with CKD (approximately 15% of participants had eGFR 30–60 ml/min/1.73 m2); there was no relationship between treatment effect and kidney function, and renal function trajectories were similar for patients in the vericiguat and placebo arms of the trail.52
Vasodilatory therapy using combined hydralazine and isosorbide dinitrate have shown mortality and morbidity benefits, particularly in people of African–American ancestry. There are signals from trials suggesting that African–American patients respond better to vasodilator therapy and the seminal African American HF Trial demonstrated that combined hydralazine and isosorbide dinitrate was associated with improved survival in black patients compared to standard care (HR 0.57; p=0.01).53
In the US, combined hydralazine and isosorbide dinitrate has been licensed for use in self-identifying black patients although in Europe such a combination is rarely used despite recommendations in ESC guidelines.54
In clinical practice, this combination is often used in patients with LVSD who are unable to have RAASis or as an add on-therapy when it is not possible to uptitrate RAASis.
Finally, patients with HF and CKD have less use and lower doses of therapies associated with prognostic benefits including ACEis/ARBs and MRAs.5 There are ongoing investigations, such as the LIFT study, for the utility of potassium binders, such as sodium zirconium cyclosilicate, to mitigate the risk of hyperkalaemia associated with RAAS inhibition (EudraCT number: 2020-002946-18).55
Renal Replacement Therapy in Heart Failure Patients
For patients with end-stage renal failure and HF, peritoneal dialysis (PD) is preferred over extracorporeal HD because of lower intra-dialytic haemodynamic shifts. PD puts less pressure on the myocardium, resulting in lower periods of myocardial ischaemia in PD.
Patients receiving PD have a better response to diuretic therapy and slower decline in kidney function than those receiving HD.56
There are also practical and logistical benefits to PD over HD in terms of time requirements, access and cost.
Device Therapy and Chronic Kidney Disease
There is strong evidence for the use of cardiac devices such as cardiac resynchronisation therapy (CRT) and ICDs in HF with EF <35% in terms of improved symptom control, reduction of sudden cardiac death (SCD), mortality and hospitalisation rates.
In the context of CKD, decisions around device therapy must be informed regarding potential difficulties with HD (such as vascular access and subclavian stenosis) and risk of infection.
Cardiac Resynchronisation Therapy
Studies, such as MIRACLE, CARE-HF, RAFT-HF and COMPANION-HF, have shown clear benefits of CRT in select patients in terms of symptoms, quality of life, hospitalisation and risk of death.57,58
In the context of coexisting CKD, 43% of the participants in the RAFT-HF study had CKD stage 3 and the study found no significant interaction between baseline renal function and treatment effect.59 Post-hoc analysis of the MIRACLE study suggested that renal function improved in patients with CKD stage 3 who received CRT compared to controls.60
Further evidence supporting the effectiveness of CRT in patients with CKD has been obtained from an inverse-probability weighted analysis of a large American Medicare database.61 This analysis of 10,946 patients with CKD stages 3–5 (including patients receiving dialysis) showed a reduced risk of HF hospitalisations or death in those receiving CRT-D (cardiac resynchronisation therapy with defibrillator) versus ICD alone (HR 0.84; 95% CI [0.78–0.91).61 A specific study looked at CRT-D in 73 patients with stage 4 CKD patients with LV ejection fraction (LVEF) <35% showed improved renal function (25 ± 4 to 30 ± 9 ml/min/1.73 m2; p=0.04) and survival (HR 0.51; 95% CI [0.27–0.98)] compared to ICD.62
ICD
Patients with combined HF and CKD are at particular risk of SCD, likely related to high rates of electrolyte disturbances, coronary artery disease and MIs.63
Two large RCTs have demonstrated mortality benefits in patients with severe LVSD who were randomised to receive a prophylactic ICD versus amiodarone or placebo (SCD-HeFT trial) or versus usual care (MADIT-II).64,65
In the MADIT-II study, 1,232 patients with previous MI and EF <30% were randomised to receive either an ICD or standard medical therapy.64 Approximately 40% of these patients had evidence of at least CKD stage 3a with 80 patients (17%) having an eGFR <35 ml/min/1.73 m2. While defibrillator therapy was associated with a survival benefit in the main analysis (all-cause mortality RR 32%; p=0.01; SCD RR 66%; p<0.001), there was no benefit found for patients with more advanced renal impairment (eGFR <35 ml/min/1.73 m2).66
A meta-analysis has confirmed that the survival benefit of ICD implantation is found only in patients with an eGFR >60 ml/min/1.73 m2.67 Furthermore, defibrillator insertion in patients on HD was associated with high infection rates, and a recent RCT failed to show any significant clinical benefit.68–70
However, there are studies supporting the use of ICD in secondary prevention for patients receiving haemodialysis who survive a cardiac arrest.71,72
Subcutaneous ICDs (S-ICD; EMBLEM, Boston Scientific) are a suitable alternative to transvenous ICDs and are particularly attractive for use in patients with CKD, who frequently have vascular access issues.
Koman et al. reported data from the follow-up of 18 HD patients and 78 non-HD patients with an implanted S-ICD. HD patients implanted with an S-ICD had similar procedural outcomes and inappropriate shock frequency.73 All appropriate shocks were successful in terminating ventricular tachyarrhythmias in both groups. There was no device or bloodstream-related infection in HD patients, compared to nearly 7% in the non-HD group.73 This seemingly paradoxical difference did not reach statistical significance. Similar results were presented by El-Chami et al.74
Leadless Pacemakers
Patients with HF often require pacing for symptomatic bradycardia, and the risk of symptomatic bradycardia is particularly high in patients with concomitant CKD.75
Wireless devices may offer a novel strategy to overcome difficulties associated with vascular access in patients with CKD. This technology is appropriate when a low burden of pacing is expected; otherwise, CRT is preferred.
Leadless devices such as the Micra (Medtronic) device have been used successfully in patients with symptomatic bradycardia and demonstrated good efficacy with lower rates of complications compared to historical control groups.76 However, this technology can provide only right ventricular pacing and currently tends to be reserved for patients with an EF >50% if there is a pacing indication.
In patients with a LVEF of 36–50% and atrioventricular (AV) block who have an indication for permanent pacing and are expected to require ventricular pacing >40% of the time, techniques that provide more physiologic ventricular activation (CRT or His bundle pacing) are reasonable in preference to right ventricular pacing to prevent HF.77,78 A study looking at Micra implantation in 201 patients receiving HD demonstrated good sensing, pacing thresholds and safety profile (including infection rates) comparable to other populations.75
There has been recent development of a leadless cardiac resynchronisation therapy. The WiSE (Wireless Stimulation Endocardially) CRT is an emerging technology, delivering wireless LV endocardial pacing as an alternative to conventional epicardial LV pacing through the coronary veins.79
It comprises a battery, an ultrasound transmitter and a receiver electrode, and is combined with a pre-existing right ventricular (RV) pacing device. The transmitter synchronises with the RV pacing pulse and immediately transmits ultrasound energy to a tiny receiver electrode implanted on the LV endocardial surface.79 The receiver electrode converts ultrasound energy into electrical energy, providing LV stimulation, resulting in simultaneous biventricular pacing.79
It is not available for clinical use yet but potentially could be useful in patients with advanced CKD at stages 4–5, who are on HD and who have venous access issues where there are concerns about a high risk of infection. The ongoing SOLVE-CRT trial is assessing the safety and efficacy of this technology (NCT02922036). However, patients with CKD stage 4–5 and on HD have been excluded.80
Carabelli et al. published their experiences in a case series (n=8) where totally leadless CRT was successfully delivered with a combination of two technologies (Micra and WiSE-CRT).81 All implanted WiSE-CRT devices could successfully detect the Micra pacing output and delivered synchronous LV endocardial pacing. QRS duration reduction and improvements in LVEF were found in all patients (28.43 ± 8.01% versus 39.71 ± 1.89%; p=0.018).81
Conclusion
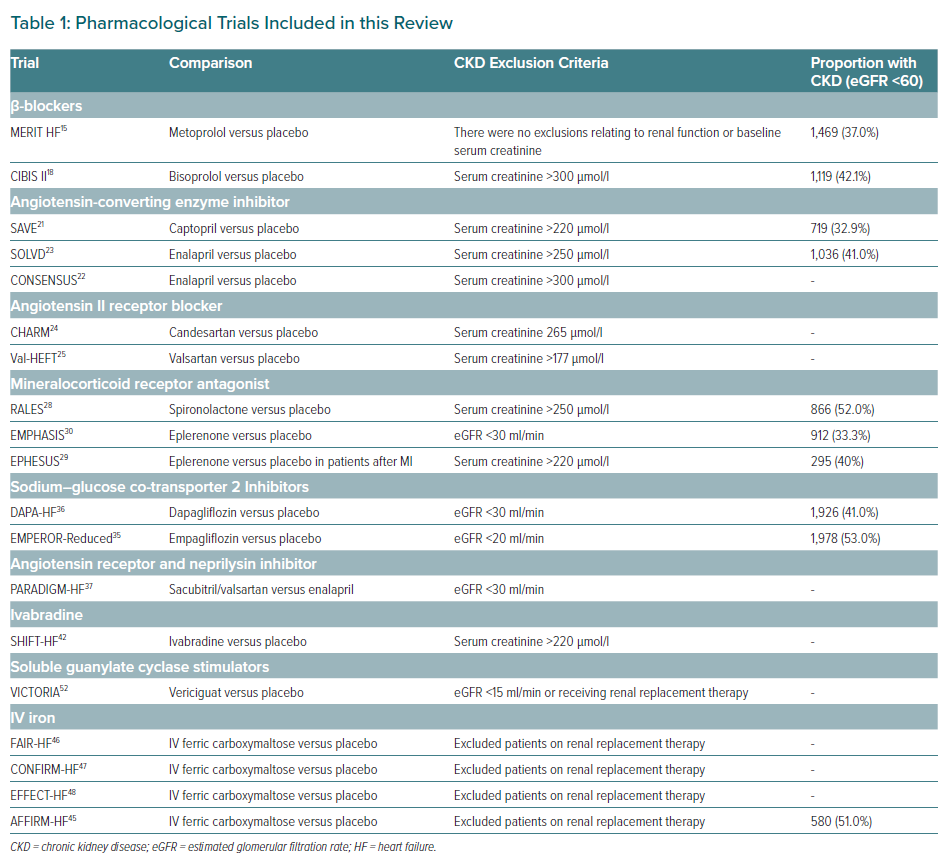
There is a pressing need to adapt the HF armamentarium and current clinical practice to meet the growing number of patients presenting with both HF and CKD. At present, existing HF therapies, such as ARNIs (in replacement of RAASis), β-blockers, RAASis, MRAs and SGLT2is, should be the cornerstone pharmacological agents for treating HF in patients with CKD stage 1–3 (Table 1).
However, these existing therapies are frequently omitted because of concerns about hyperkalaemia and renal side-effects. Given the significant prognostic benefits of RAASi, these potential side-effects should not be a barrier to ACEi/ARB/ARNI initiation for patients with well-controlled CKD at stages 1–3.
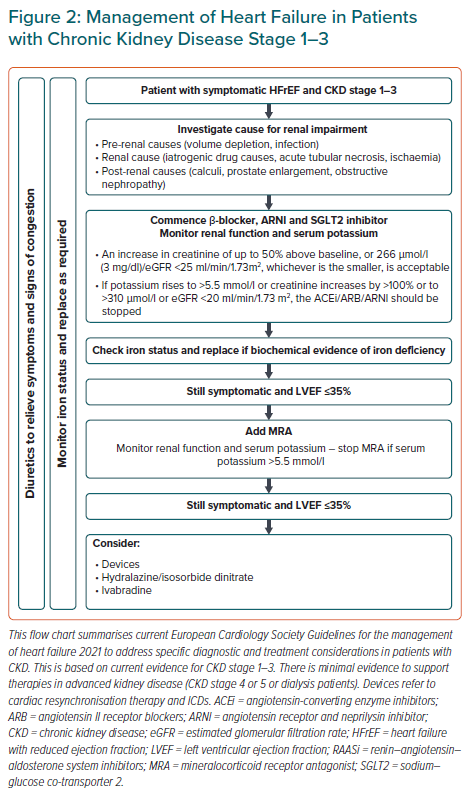
Judicious monitoring of renal function and side effects are warranted given the clear prognostic benefits of these medications in patients with HF and CKD at stages 1–3. The ESC has produced a helpful document on what to do in case of worsening renal function and hyperkalaemia (Figure 2).8
There is a growing evidence base for novel therapies including cardiac devices in patients with HF and CKD. However, it is unclear what context and combination of therapies provide optimal management for HF and CKD. Incorporating specialist cardiology and renal health professionals into multidisciplinary care pathways would be the optimal approach to improve the quality of care provided to patients with HF and CKD.