Non-alcoholic fatty liver disease (NAFLD), also named metabolic dysfunction-associated fatty liver disease (MAFLD), is a progressive disease spectrum encompassing simple steatosis, non-alcoholic steatohepatitis, fibrosis and cirrhosis.1 Hepatic steatosis refers to ectopic deposition of triglycerides in the liver. It is necessary to exclude secondary hepatic fat accumulation (heavy alcohol consumption, virus, autoimmune disease) in a diagnosis of NAFLD.2
NAFLD continues to be an undetected pathology despite its status as the most common liver disease, affecting more than 32% of adults worldwide.3 The prevalence varies by race and ethnicity, being highest in Hispanic people, men and older individuals.4 Over the last decade, the rate of this pathology has been increasing exponentially in Western industrialised countries, where the cardiovascular (CV) risk factors are common.5
Nevertheless, the disease continues to be overlooked. The main reason is that most patients with NAFLD are asymptomatic.6 Although some patients may complain of fatigue, malaise or abdominal discomfort, this pathology is usually suspected with the finding of hepatomegaly on physical examination due to fatty infiltration of the liver, or incidentally on laboratory tests (elevated liver enzymes) or on abdominal imaging.2,4
The new term MAFLD emphasises the bidirectional relationships involved in this disease, i.e. the possible involvement of fatty liver disease in patients with type 2 diabetes (T2D) and CV disease (CVD) or its risk factors, and, in turn, the possibility of these diseases in patients with NAFLD.7 Some of the well-established risk factors for MAFLD include obesity, T2D, dyslipidaemia and metabolic syndrome.7 Sharing several cardiometabolic risk factors, NAFLD and CVD have a strong association.8 Various large population-based studies have identified NAFLD as an independent risk factor for CVD.3 Currently, CVD is the most common cause of death in patients with NAFLD.9,10
Therefore, although NAFLD has traditionally been interpreted as a liver disease with a high risk of liver-related complications, and most patients have usually been referred to gastroenterologists and hepatologists, patients also have an increased chance of developing CVD, and the landscape of CV risk in this clinical setting is continuously evolving.9 Given the pandemic-level rise of NAFLD and its association with poor CV outcomes, the question of how to manage NAFLD successfully, in order to reduce the burden of associated incident CV events, is both timely and highly relevant. In this narrative review we aim to summarise the current knowledge of the association between NAFLD and CVD, and discuss available treatment options for modification of CVD morbidity and mortality in patients with NAFLD.
NAFLD and Increased Risk of CVD: Pathophysiological Mechanisms
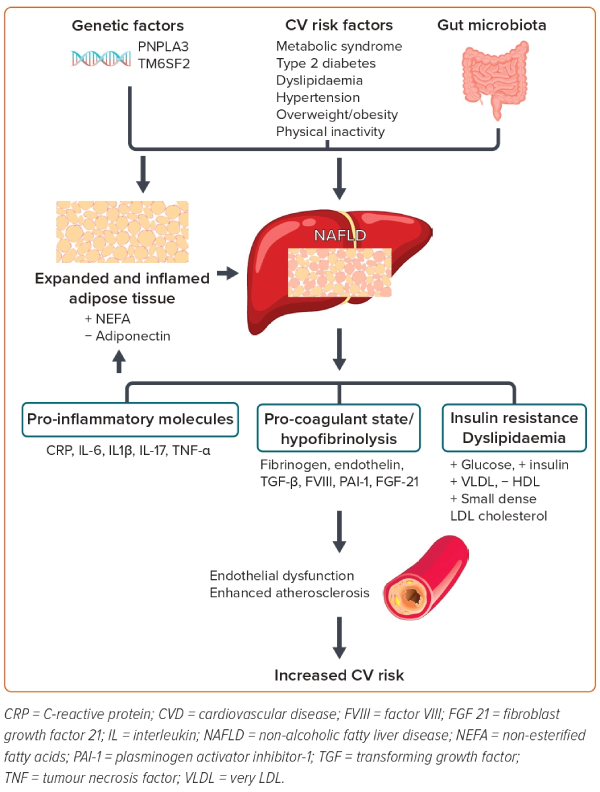
The pathophysiology behind the association of NALFD with other CVDs remains incompletely understood. There are different theories. The most accepted theory implicates insulin resistance as the key mechanism leading to hepatic steatosis, and perhaps also to steatohepatitis. Buzzetti et al. best explain the ‘multiple-hit’ hypothesis for NAFLD pathogenesis as the cumulative effect of various insults.11 Some of the mechanisms by which NAFLD increases CVD risk include systemic inflammation, endothelial dysfunction, hepatic insulin resistance, oxidative stress and altered lipid metabolism.12
NAFLD, especially in its necroinflammatory form (non-alcoholic steatohepatitis; NASH), may also cause atherogenic dyslipidaemia. In addition, there is an increase of pro-coagulant factors, such as fibrinogen, plasminogen activator inhibitor-1 and tumour growth factor, which all increase the risk of atherosclerosis.6,12
Inflammation is also crucial in the pathogenesis of NAFLD. NAFLD is considered to generate chronic sub-clinical inflammation and is associated with many markers of inflammation. Increased CV risk has been linked to increased levels of inflammatory cytokines and markers such as tumour necrosis factor-α (TNF-α), interleukin-6 (IL-6), C-reactive protein (CRP) and fibrinogen. Oxidative stress may also play a role. This stress is thought to trigger changes in endothelial function leading to formation and deposition of oxidised LDL cholesterol in the sub-intimal space.6
It is also possible that NAFLD and CVDs share a common inherited predisposition, which further influences the CV risk in these patients.12,13
Therefore, the underlying mechanisms linking NAFLD to CVD are very complex and involve several different pathways simultaneously. Further research is required to identify other specific mechanisms by which NAFLD and NASH may contribute to the development and progression of CVD (Figure 1).
NALFD and Atherosclerosis Risk
The available evidence not only demonstrates the strong association between NALFD and CVD, but also supports the view that NAFLD may increase the risk of incident CV events.14 Strong evidence links NAFLD to objectively assessed subclinical atherosclerosis, including coronary artery calcium score in adults and adolescents, as well as to an increased prevalence of clinically manifest CVD both in the general population and in different patient groups.15 Therefore, NAFLD serves as an important atherogenic risk factor and reemphasises the role of early risk evaluation and prophylactic intervention measures to preclude progression to clinical CVD in NAFLD.
Several recent meta-analyses have shown that NAFLD has a significant independent association with subclinical atherosclerosis, and that the presence of NAFLD conferred a remarkably higher risk of increased carotid artery intima–media thickness or plaques, arterial stiffness, coronary artery calcification, and endothelial dysfunction with ORs of 1.74 (95% CI [1.47–2.06]), 1.56 (95% CI [1.24–1.96]), 1.40 (95% CI [1.22–1.60]), and 3.73 (95% CI [0.99–14.09]), respectively, and concluded that patients with NAFLD might benefit from screening and surveillance of early atherosclerosis, which would facilitate the prediction of potential CVD burden, risk stratification and appropriate intervention in the long term.16,17
Another meta-analysis of six studies with 25,837 patients found that patients with NAFLD had a significantly higher risk of clinical CV events compared with controls (RR 1.77; 95% CI [1.26–2.48]; p<0.001).18 The association remained consistent for subgroups with clinical coronary artery disease (RR 2.26; 95% CI [1.04–4.92]; p<0.001) and ischaemic stroke (RR 2.09; 95% CI [1.46–2.98]; p<0.001). The risk of CV mortality was also increased in the NAFLD group (RR 1.46; 95% CI [1.31–1.64]; p<0.001).
The severity of NAFLD is also associated with a higher incidence of CVD risk factors such as diabetes and hypertension, and higher CV risk.19,20 A clinical diagnosis of NAFLD and particularly NASH should lead the clinician to carry out targeted CVD risk assessment and management. This has relevance for clinicians who see patients in the primary care or hepatology clinic, where the initial presenting problem may be abnormal liver function or obesity rather than CVD risk reduction.21,22
Management of NAFLD: Measures Aimed at Reducing Both Liver Disease and CV Risk
NAFLD, despite its global prevalence, is still a therapeutic challenge for modern medicine. The question of how to manage NAFLD successfully, in order to reduce the burden of associated incident CV events, is both timely and highly relevant.
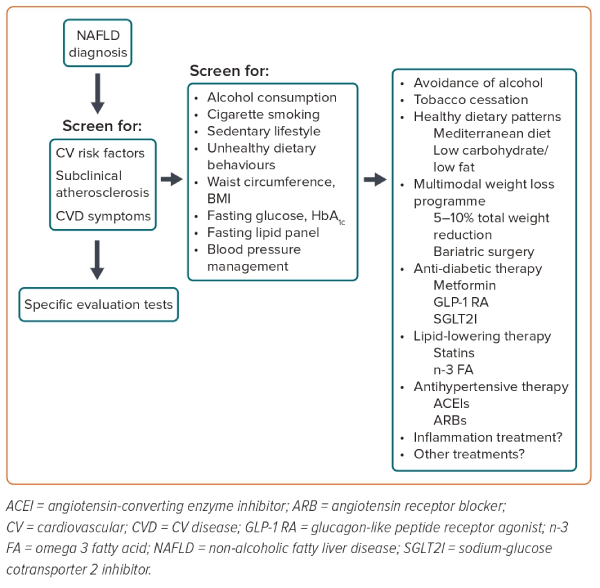
A tailored multistep treatment approach has been proposed for the management of NAFLD as an effective tool to reduce the risk of CV disease in these patients.23 The first fundamental step is to achieve favourable lifestyle changes by means of an appropriate diet, physical exercise and smoking cessation. Further steps that may lead to bariatric surgery should, however, be primarily reserved for patients with NASH- or NAFLD-associated high-risk metabolic comorbidities, such as obesity, dyslipidaemia, hypertension and T2D (Figure 2).21,23
Non-pharmacological treatment
Lifestyle intervention is the key therapeutic intervention for patients with NAFLD.2,4,24 Dietary modification, increased physical activity, weight loss and alcohol avoidance are strongly recommended.
Diet
Body mass has a direct influence in the majority of NAFLD cases. Due to this fact, patients are persuaded to change their diet and lifestyle. Lifestyle interventions that encourage calorie restriction to induce weight loss and disease regression are the cornerstone of NAFLD management.2,4 Changing dietary composition is also effective for CVD and T2D risk reduction in patients with NAFLD. Hypocaloric diets have been proven to reduce intrahepatocellular lipid content. A study of NAFLD demonstrated that time-restricted feeding reduces triglycerides levels after 12 weeks as compared with the control group.25 The Mediterranean diet has also been shown to reduce hepatic fat and to improve insulin sensitivity independently of exercise and weight loss. Research has shown that greater adherence to the Mediterranean diet is effective for NAFLD prevention and management and that those randomised to a Mediterranean diet intervention had reduced CVD.26
Avoidance of Alcohol
Alcohol consumption has been extensively studied as a modifiable risk factor for CVDs. Excessive alcohol consumption (>60 g/day in men and >40 g/day in women) is a well-known contributor to mortality and CVD burden. A large number of observational studies reported beneficial associations of low to moderate alcohol consumption (up to 60 g/day in men and up to 40 g/day in women) with CVD. This results in a characteristic biphasic, J-shaped risk profile in which, for low to moderate alcohol consumption, a lower CVD risk is observed compared with abstinence and excessive drinking. Nevertheless, given that most of the evidence of the protective effects of low–moderate alcohol consumption against CVD originates from observational studies, the question remains whether this effect is truly causal or merely a result of different forms of bias inherent in observational study designs.27 This protective effect has not been demonstrated specifically in patients with NAFLD. Studies indicate that any alcohol consumed by patients with established NASH enhances disease progression and therefore should be completely avoided.28
Physical Activity
Exercise is another essential lifestyle intervention for the management of NAFLD and to reduce CVD. An increase in sedentary time may lead to a predisposition towards NAFLD. Increased breaks in sedentary time are reported to be beneficial for glucose and fatty acid metabolism, and obesity control.12 A meta-analysis reported that exercise reduces hepatic fat content, with little or no weight loss.29 Vigorous activity is much more beneficial for NAFLD and fibrosis than light activity. Current clinical guidelines from the American College of Sports Medicine, American Gastroenterology Association, and European Association for the Study of the Liver all agree that at least 150 min/week of moderate-intensity aerobic activity, such as brisk walking or light cycling, is recommended to all patients with NAFLD and NASH.10,30 A recent meta-analysis of 14 studies confirms that exercise leads to clinically meaningful reductions in liver fat for patients with NAFLD, and, independent of weight loss, the team found that exercise training was 3.5-fold more likely to achieve clinically meaningful treatment response (≥30% relative reduction in MRI-measured liver fat) than standard clinical care.31
Weight Loss
Currently, weight loss is the most effective treatment for NAFLD, even in the minority of patients with NAFLD who do not have obesity, and is recommended in all guidelines.32 Even modest weight loss (5–10%) is associated with significant benefits. In particular, a weight loss of at least 10% has been shown to resolve not only steatohepatitis, but also fibrosis in liver tissues.33 A 5–10% bodyweight loss could be a challenging goal for many patients if they only do exercise or follow a diet. Therefore, bariatric surgery is an option for some patients who cannot achieve the goal of losing 0.5–1 kg/week.34 Recently, high-level evidence has shown that bariatric surgery has an impact on NAFLD, with an 88% improvement in steatosis and 30% in fibrosis.35 A systematic review and meta-analysis was performed to compare the impact of Roux-en-Y gastric bypass (RYGB) and sleeve gastrectomy (SG) on NAFLD and NASH.35 The authors found that the NAFLD activity score was significantly improved after both procedures, as were the biochemistry results. No difference was found between RYGB and SG regarding the histopathological outcomes. The authors concluded that SG and RYGB were equivalently effective for treating NAFLD and NASH.35
Pharmacological Treatment
In some cases, diet and lifestyle measures cannot be successfully or sustainably implemented. Pharmacological treatment is an option when non-pharmacological treatment fails, or when the patients already have advanced disease. The majority of drugs are used to control CV risk factors and to help people to lose weight. Liver-targeted therapy, especially for NAFLD, is limited.36
Hypertension Treatment
Angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) have been noted as a promising medication given that the renin–angiotensin–aldosterone system (RAAS) is involved in the pathogenesis of both NAFLD and CV pathologies.37 The RAAS, which has a central function in the physiology of blood pressure, is reported to be associated with inflammation and fibrosis in NAFLD. Moreover, RAAS blockers, including ACEIs and ARBs, have been shown to exert protective effects against liver fibrosis.38 These effects are due to suppression of the transformation of hepatic stellate cells into hepatic myofibroblasts in response to elevated expression of pro-inflammatory cytokines and reduced expression of tissue growth factors, angiotensin II type-1 receptor (AT1R) and transforming growth factor (TGF)-β1.39 Although several studies indicated beneficial effects of these drugs, the current evidence is insufficient to support the efficacy of RAAS blockers in managing fibrosis in NAFLD patients.
Diabetes Treatment
As we have noted, there is a bidirectional relationship between NAFLD and T2D. The liver plays a vital role in the pathophysiology of both diseases because it is involved in the development of insulin resistance, which in turn results in NAFLD and T2D. In addition, there are common management options for the two diseases.40
It needs to be considered that some glucose-lowering drugs promote adipogenesis and the accumulation of epicardial fat (sulfonylureas, insulin), whereas other anti-diabetic drugs (e.g. glucagon-like peptide-1 receptor agonist [GLP-1 RA] and dipeptidyl peptidase type 4 [DPP-4] inhibitors) reduce the accumulation of ectopic fat but do not reduce inflammation.40 In contrast, both metformin and sodium-glucose cotransporter 2 inhibitor (SGLT2I) improve both processes. SGLT2I and GLP-1 RA also reduce CV events; therefore, both treatments are promising in this type of patient.41–43 This suggests that a number of pharmacological agents used to treat diabetes, such as pioglitazone, liraglutide or semaglutide, and potentially SGLT2I, alone or combined, may offer a therapeutic option for patients with T2D and NASH. A dual-treatment strategy for patients with T2D and coexisting NAFLD, based on the efficacy of pioglitazone, GLP-1 RAs and/or SGLT2I as monotherapy in placebo-controlled trials, awaits testing as combination versus NASH in this population.43
Metformin
Metformin is used as a first-line therapy for T2D. Clinical studies have reported that when metformin is used for the treatment of T2D in people with obesity, it significantly reduces body weight, limb, android and gynoid fat mass while increasing the total lean mass. Moreover, metformin may correct several components of metabolic syndrome such as impaired glucose tolerance and lipid metabolism disturbance. Metformin is also frequently prescribed off-label to patients with this disease, because the activation of AMP-activated protein kinase (AMPK) has been shown to be associated with a plethora of beneficial effects, including decreased oxidative stress and inflammation of the liver.44 Metformin has also been shown to result in a modest improvement in biochemistry in patients with NAFLD not responding to lifestyle intervention. It also improves alanine aminotransferase (ALT) levels and liver histology in 25% of patients with NAFLD, which is likely to be attributable in part to weight loss.20 Currently, the evidence is low, therefore metformin is not recommended as a treatment for liver disease in adults with NAFLD.45
Thiazolidinediones
The discovery of peroxisome proliferator-activated receptor-γ (PPAR-γ) in adipose tissue produced a step change in adipose tissue research. PPARs are a group of nuclear receptor proteins that function as transcription regulators, and PPAR-γ heterodimerises with retinoid X receptor and binds to specific DNA sequences to regulate adipocyte differentiation and function, lipid metabolism and inflammation.46 Glitazones are selective activators of PPAR-γ, and they act by redistributing fat from ectopic tissues to the adipose tissue, by increasing levels of adiponectin. They all contribute to the reduction of insulin resistance.47
Pioglitazone is the most potent insulin sensitiser currently licensed for the treatment of T2D, and this drug has the strongest evidence base for the treatment of NALFD. Moreover, pioglitazone was also associated with a 28% decrease in the incidence of MI and a 47% decrease in the incidence of ischaemic stroke.48
In a recent systematic review of 10 randomised control trials involving 887 participants, the authors found that pioglitazone consistently improved histological parameters and normalised liver transaminases, although evidence supporting the benefits of other drugs in this class was minimal.49 Unfortunately, this drug has attendant side-effects such as weight gain and fractures, limiting its widespread use; hence, careful selection of candidates is imperative.47–49
Glucagon-like Peptide
Beyond their anti-hyperglycaemic effect and their surprising role in cardio- and nephroprotection, GLP-1 RAs have been shown to have a significant impact on body weight and clinical, biochemical and histological markers of fatty liver and fibrosis in patients with NAFLD. Therefore, GLP-1 RAs could be useful for the treatment of both T2D and NAFLD.50
In addition to glycaemic control in T2D, GLP-1 RAs have been shown to be extraordinarily effective in the prevention and treatment of its complications. In particular, they have been shown to effectively reduce the rate of major adverse CV events (MACEs, such as MI and ischaemic stroke) and related mortality. Overall, GLP-1 RA treatment is associated with a 9–16% reduction in CV events and all-cause mortality. For this reason, GLP-1 RAs have become the first-line treatment for patients with T2D with evidence of atherosclerotic disease and/or a previous CV event.50,51
GLP-1 is an intestinal hormone released from the foregut in response to food consumption. GLP-1 has a glucose-lowering action by its glucose-dependent ability to stimulate insulin and suppress appetite. This metabolic effect has a beneficial impact on insulin resistance and weight loss.52 Also, the GLP-1 receptor is present in hepatocytes; therefore, GLP-1 agonists may have a direct effect on the liver, reducing fat accumulation by activating macroautophagy and chaperon-mediated autophagy.53
Liraglutide
Liraglutide is glucagon-like peptide that is highly effective for the treatment of T2D and has proven cardiovascular benefit.41 This drug has been the GLP-1 RA most broadly studied in NAFLD. The LEAN trial showed that patients with NASH treated with liraglutide had reduced progression of fibrosis.54 However, confirmatory studies using liraglutide are needed, to determine the extent to which improvement may be attributable to mechanisms beyond weight loss.
Semaglutide
Semaglutide has been clinically approved for the treatment of T2D. Observations from Phase III clinical trials, such as SUSTAIN and PIONEER, suggest the antiobesity potential of semaglutide.55.56 It has also been approved by the Food and Drug Administration for the management of obesity. Given that semaglutide improves insulin resistance via the insulin signalling pathway and reduces body weight, both of which are responsible for the progression of NALFD, it has been considered a good option for the treatment of patients with this pathology.57
Semaglutide has also been shown to be hepatoprotective in animal and human trials, alone or in combination. Thus, for example, in a recent Phase II, open-label, proof-of-concept trial in which 108 patients with NASH were randomised to 24 weeks’ treatment with semaglutide 2.4 mg once weekly as monotherapy or combined with once-daily cilofexor (farnesoid X receptor agonist) and/or once-daily firsocostat 20 mg (acetyl-coenzyme A carboxylase inhibitor), combined treatment resulted in additional improvements in liver steatosis and biochemistry versus semaglutide alone.58
GLP-1 and Glucose-dependent Insulinotropic Polypeptide
Tirzepatide
Glucose-dependent insulinotropic polypeptide (GIP) receptor agonists (GIP RAs) are effective in stimulating insulin secretion and favouring peripheral glucose uptake; and in animal models GIP RAs potentiate GLP-1-induced weight loss and reduce food intake.50
Recent results from the SURPASS trials have promising implications for the management of obesity, diabetes and NALFD.59 These studies show that tirzepatide at weekly doses ranging from 5 mg to 15 mg induce a significant dose-dependent reduction in body weight, ranging from 8% to 13%, together with a marked improvement in glucose metabolism and cardiometabolic risk factors. Based on this evidence, tirzepatide is becoming an attractive therapeutic option for NAFLD or NASH, particularly in individuals with coexisting T2D and obesity.60
Cholesterol-lowering Drugs
High levels of cholesterol are generally considered to be associated with atherosclerosis and CVD; however, excess cholesterol accumulation in various tissues and organs has been shown to play a critical role in the pathogenesis of multiple diseases, including NAFLD and NASH, which may be targeted by specific pharmacological treatment.61 Statins are the first step to achieve the LDL cholesterol goal and they play an essential role in the primary and secondary prevention of CVD. As noted earlier, CVD is the main cause of death in patients with NAFLD.14
Experimental and clinical studies have shown that the effects of statins go beyond their CV-protective ability and consist of anti-inflammatory, anti-thrombotic and anti-fibrotic properties, and may thus inhibit the progression from simple steatosis to fibrosis and NASH.61 However, there are limited high-quality data with histological liver endpoints showing that statin use improves NASH; hence, statins are not currently recommended for NASH treatment. We use statins only in patients with NAFLD to achieve CV endpoints and to prevent major CVD.62
Moreover, prescribing statins to patients with chronic liver disease often raises the issue of hepatotoxicity, given that statins are metabolised in the liver by CYP450 isoenzymes. However, statin use was safe even in those with NAFLD and elevated liver enzymes, meaning that statins might be targeting both the genesis (or worsening) of NAFLD and the risk of coronary artery disease, which is increased in NAFLD patients.
A meta-analysis showed that the prevalence of elevated transaminases in patients using statins or other lipid-lowering medication is not significantly different from that in individuals using placebo.63,64 Therefore, convincing evidence regarding the safety of statins in NAFLD patients is available, and statins can be used safely and are not contraindicated in patients with NAFLD who have normal liver function.
The evidence concerning the hepatoprotective effects of statins is segmented and inconclusive. Nevertheless, in a recent multidimensional study on the potential benefits and mechanism of action of statins in NAFLD, statin use was associated with a lower prevalence of NASH and fibrosis and might prevent NAFLD.64 This may be partially attributed to the anti-lipid and anti-inflammatory characteristics of statins. The study included a meta-analysis of seven studies and indicated a non-significant inverse association for statin use with NAFLD (pooled OR 0.69; 95% CI [0.46–1.01]) and significant inverse associations with NASH (pooled OR 0.59; 95% CI [0.44–0.79]) and with fibrosis (pooled OR 0.48; 95% CI [0.33–0.70]). With regard to in vitro experiments, statins significantly reduced lipid droplet accumulation in human liver organoids and downregulated expression of pro-inflammatory cytokines in macrophages.64
Inflammation Treatment Targets: Other Drugs
Metabolic inflammation is a crucial component in the pathogenesis of obesity, T2D, CVD and NAFLD, and it is characterised by a systemic, low-grade inflammatory process in response to several non-infectious factors such as unhealthy dietary habits, especially a high-fat diet.65,66 This so-called lipotoxicity activates inflammatory pathways and components of the immune system and has been observed in the liver, arterial vessels, adipose tissue, muscle, pancreas and the central nervous system. Various compartments, such as the liver, the gastrointestinal tract and adipose tissue, are significant sources of pro-inflammatory drivers, including TNF, IL-6, IL-1β, CRP, fibrinogen and fetuin A.
Several drugs have been shown to act on inflammatory pathways and may be key also in the management of liver diseases.66,67
In atherosclerosis, the adipose tissue that surrounds blood vessels has been established as metabolically active and an important player in the inflammatory process of atherosclerosis. Perivascular adipose tissue adipocytes secrete adipokines that act in a paracrine or vasocrine manner. In a healthy person, perivascular adipose tissue secretes adipokines that promote vasodilation and have anti-inflammatory effects. In the unhealthy state, perivascular adipose tissue secretes pro-inflammatory adipokines and cytokines, which affect the surrounding vasculature and promote atherosclerosis.68
Liver inflammation in NAFLD may be regarded as a multidirectional process in which inflammatory stimuli may arise from extrahepatic tissues such as adipose tissue and the gut and inside the liver. Therefore, therapeutic antagonism of pro-inflammatory cytokines leads to improvement of hepatic steatosis, liver inflammation and fibrosis.67
Cytokines
It is widely accepted that cytokines play a critical role as mediators of inflammation, fibrosis and cirrhosis in NAFLD.68 As we have noted, several mediators of inflammation are involved in the development and progression of NAFLD, such as IL-1β, IL-6, TNF-α and CRP. Cytokines may play a key role in the pathogenies of NAFLD by stimulating hepatic inflammation, steatosis, cell apoptosis and necrosis and by inducing fibrosis.68 Moreover, these inflammation mediators can be used as biomarkers to assess the severity and predict the outcome of NAFLD.69
Tumour Necrosis Factor-α
Given that TNF-α is one of the causal factors contributing to NASH progression, a combination of therapeutic modalities, including TNF-α-based therapies, may lead to the resolution of NASH via multiple pathways and thus generate clinical benefits. Anti-TNF-α therapy has been widely used in the treatment of chronic inflammatory diseases such as uveitis.70 However, there have been no approved therapies so far for NASH. Among the various putative drug targets, TNF-α is a possible therapeutic target of NASH, as shown in preclinical studies of anti-TNF-α strategies.70 Thus, for instance, animal studies of thalidomide and infliximab have shown substantial reduction in steatosis.71 However, further studies in humans are necessary to clearly demonstrate and confirm these preliminary results.
Transforming Growth Factor-β
TGF-β signalling mechanisms play a central role in maintaining normal liver homeostasis. TGF-β1, one of the three isoforms of the TGF-β family, plays a significant role in different stages of chronic liver conditions. TGF-β1 promotes hepatic stellate cell activation, which further contributes to the progression of NAFLD.72 Galunisertib is a promising anti-fibrotic TGF-β receptor type I kinase inhibitor that inhibits SMAD2 phosphorylation and blocks collagens deposition, and is a potential candidate for the treatment of liver fibrosis.73
Interleukin-11
IL-11 is important for fibrosis in NASH. Hepatocytes express IL-11 receptor-α and secrete IL-11 in response to lipid loading. Autocrine IL-11 activity causes hepatocyte death through NADPH oxidase 4 (NOX4)-derived reactive oxygen species, activation of extracellular signal-regulated kinases, c-Jun N-terminal kinases and caspase-3, impaired mitochondrial function and reduced fatty acid oxidation. Paracrine IL-11 activity stimulates hepatic stellate cells and causes fibrosis.74 Neutralizing antibodies that block IL-11 signalling reduce fibrosis, not the neutralisation of antibodies that block IL-11 caused reduction in fibrosis, steatosis, hepatocyte apoptosis and inflammation in mice.66 Therefore, targeting IL-11 to reverse liver fibrosis may be a beneficial therapy for NAFLD.
Cenicriviroc
Cenicriviroc (CVC) is a novel, orally administered and potent C chemokine receptor type 2 (CCR2) and type 5 (CCR5) antagonist, which is currently in clinical development for the treatment of liver fibrosis in adults with NASH. It blocks overactive inflammatory signalling and stellate cell activity, thereby targeting both inflammation and fibrogenesis.75 CVC was shown to have antifibrotic effects in animal models, and blocked CCR2 and CCR5 in the Phase IIb CENTAUR study in adults with NASH and liver fibrosis. CVC treatment improved fibrosis, the histological feature consistently linked with clinical outcomes in NAFLD.76 Among the target population in the Phase IIb study, that is, subjects with NASH and stage F2 or F3 liver fibrosis, 28% of CVC-treated subjects achieved ≥1 stage improvement in liver fibrosis without worsening of NASH compared with 16% on placebo, with a greater effect observed in those with more advanced fibrosis.77 Currently, another randomised, double-blind, placebo-controlled, multi-centre Phase III study, AURORA, aims to evaluate and confirm the efficacy and safety of CVC for the treatment of liver fibrosis in adults with NASH.75
Farnesoid X Receptor Agonist
Farnesoid X receptors (FXRs) are nuclear hormone receptors expressed in high amounts in body tissues that participate in bilirubin metabolism, including the liver, intestines and kidneys. FXRs play a critical role in carbohydrate and lipid metabolism and the regulation of insulin sensitivity. FXRs also modulate liver growth and regeneration during liver injury. Preclinical studies have shown that FXR activation protects against cholestasis-induced liver injury.78 Moreover, FXR activation protects against fatty liver injury in animal models of NAFLD and NASH.78 Some synthetic FXR agonists are being tested for the treatment of NASH, such as obeticholic acid and tropifexor, and results from preclinical and clinical studies indicate that targeting FXR is a promising treatment strategy for NASH.79,80
Other Peroxisome Proliferator-activated Receptor Agonists
PPARs are a group of nuclear receptors that are expressed in the liver, adipose tissue, heart, skeletal muscle and kidney.81 In the liver, PPAR-α upregulates several enzymes involved in mitochondrial and peroxisomal fatty acid oxidation, microsomal oxidation and ketogenesis, and therefore shifts the hepatic metabolism towards lipid oxidation. In vitro and in vivo studies have shown that PPAR-α suppresses the secretion of IL-1, IL−6 and TNF-α.82 These effects induce a reduction in inflammation and fibrosis. Elafibranor, a PPAR-α/δ agonist, was a promising treatment in Phases I and II, and improved the NAFLD activity score in a Phase IIb study of 276 patients with NASH, although the Phase III trial (RESOLVE-it) was terminated early because the drug failed to meet the primary endpoint.83 However, lanifibranor, a pan-PPAR agonist, had good results in Phase I and II testing.84 Phase III testing (NATiv3) is planned.85
Conclusion
Both NAFLD and atherosclerotic CVD are growing public health problems. Although NAFLD has traditionally been interpreted as a liver disease with a high risk of liver-related complications, currently we know that NAFLD is a risk factor for atherosclerotic CVD, which is the principal cause of death in patients with NAFLD. The multiple mechanisms linking NAFLD and CVD include inflammation, oxidative stress, insulin resistance, ectopic adipose tissue distribution, dyslipidaemia, endothelial dysfunction and adiponectin, among others. These factors not only cause NAFLD, but also accelerate the progress of atherosclerosis and the development of CVD. The clinical implication is that patients with NAFLD are at an increased risk of CVD and should be considered as candidates not only for aggressive treatment of their liver disease but also for careful monitoring and potential treatment of underlying CVD risk factors, given that many patients with NAFLD will have major CVD events and die prior to the development of advanced liver disease.
Nevertheless, despite the alarming prevalence of NAFLD, there are still limitations in knowledge and unmet needs in the management of NAFLD among medical providers. Patients with NAFLD are at increased risk of CV and hepatic diseases, but currently it is unclear how best to stratify patients into appropriate risk groups for targeted interventions, and further studies are needed to define optimal treatment strategies for the prevention of both hepatic and CV complications.