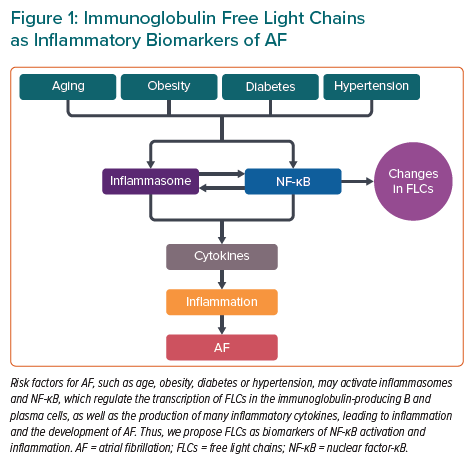
Inflammation in AF
AF is a common cardiac arrhythmia, and inflammatory mechanisms play an important role in its pathogenesis.1–7 AF is commonly found in older individuals, and type 2 diabetes, high blood pressure, obesity and genetics are important predisposing factors (Figure 1).2–4 AF increases the risk of heart failure, stroke and thromboembolism, and increases morbidity and mortality in patients with cardiovascular diseases (CVDs).5
Inflammation is perhaps the most important determinant of AF and subsequent fibrosis in MI.2 Myocardial ischaemia causes the release of various inflammatory mediators, such as interleukin (IL)-6 and C-reactive protein (CRP), which are independently associated with the development of AF. Furthermore, inflammation induced by MI leads to atrial remodelling through the activation of toll-like receptors, which are part of the innate immune system.2
Inflammatory markers such as CRP, IL-6, IL-1β, myeloperoxidase and tumour necrosis factor (TNF)-α are correlated with the progression of AF, and can predict the outcome of AF ablation.8 A meta-analysis confirmed that perioperative concentrations of CRP and inflammatory cytokines (i.e. IL-6, IL-8, and IL-10) are significantly associated with postoperative AF.9 In addition, concentrations of the chemokine monocyte chemoattractant protein-1 are also associated with an increased risk of AF and atrial fibrosis.8 Abnormal atrial histology compatible with myocarditis was found in patients with lone AF. Moreover, immune cells can be recruited from peripheral tissue to the myocardium and induce a local inflammatory response.
In line with this mechanism, macrophages and T cells have been found in the atrial myocardium of patients with AF.10 Patients with AF had higher nuclear factor (NF)-κB activity and more severe lymphomonocyte infiltration than those in sinus rhythm. These observations implicate local immunological inflammatory responses within the atrial endocardium in AF.7
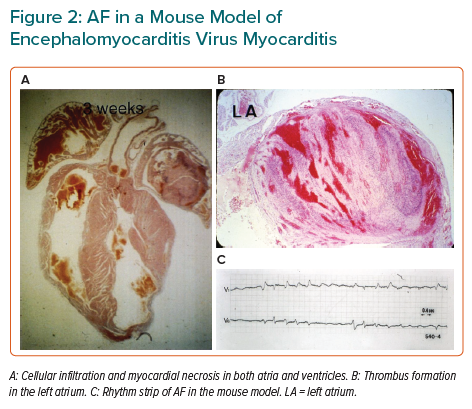
In a model of viral myocarditis in mice, serial ECGs showed various atrial and ventricular arrhythmias, as well as conduction disturbances.11 In addition, AF was observed in some mice, accompanied by cellular infiltration, myocyte necrosis in the atrial wall and thrombus formation in the atrial cavity (Figure 2).11
Inflammasome Activation in AF
Inflammasome activation contributes to the onset and progression of AF.1,5 Inflammasomes are intracellular protein complexes that activate inflammatory signalling. Inflammasomes form in response to the cellular detection of a broad range of signals, including microbial motifs, danger signals, and environmental irritants, and serve as the first line of defence against pathogens. NOD-like-pyrin-domain-containing-protein-3 (NLRP3) is an inflammasome that has been discovered in various types of cells. Inflammasomes regulate immune responses, and their activation involves priming and triggering. Elevated active caspase-1 and NLRP3 concentrations, as well as enhanced macrophage infiltration in atrial tissue, have been reported in chronic and postoperative AF, suggesting that NLRP3 inflammasome activation may contribute to AF.1
Activation of NLRP3 inflammasomes can enhance the activity of other inflammatory cytokines, such as IL-6 and TNF-α, which are related to the development of AF.9 NLRP3 inflammasome activation releases IL-1β, which can trigger the IL-6 and TNF-α expression via the IL-1 receptor (IL-1R), and the formation of active NF-κB. Increased IL-6 and TNF-α concentrations are associated with AF development in patients, as well as with adverse outcomes of AF ablation.12 High-sensitivity CRP (hsCRP) concentrations also increase with inflammasome activation (Figure 1).6
Because NF-κB is a master transcription factor controlling the expression of many inflammatory cytokines and NF-κB activation is likely the consequence of several cytokine receptors, including IL-1R and the TNFα receptor, a deleterious inflammatory signalling cycle involving multiple cytokines may exist in atrial tissue, supporting the development of AF (Figure 1).13 Post-transcriptional and post-translational mechanisms that regulate NF-κB and inflammasome signalling may play key roles in the development and persistence of inflammation in AF. It is likely that further investigations regarding NF-κB and inflammasome signalling will identify new regulatory mechanisms that may offer additional opportunities for diagnostic and therapeutic interventions (Figure 1).13
The importance of proteostasis derailment in AF has been suggested, including exhaustion of cardioprotective heat shock proteins, disruption of cytoskeletal proteins via histone deacetylases and the recently discovered DNA damage-induced depletion of NAD+ as root causes of AF promotion.8 Various studies have indicated potential cross-talk between modulators of proteostasis derailment and inflammation-induced stimuli. Emerging evidence suggests that derailed proteostasis can enhance activation of the NLRP3 inflammasome via increased protein misfolding and stress pathways in the endoplasmic reticulum in autoinflammatory diseases.14 Because of the reciprocal modulation between proteostasis derailment and inflammasome activation, mechanistic evidence of cross-talk in AF is required to provide a holistic understanding of the molecular mechanisms underlying AF.8
The recent Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) showed that the monoclonal antibody against IL-1β significantly reduced cardiovascular events. The benefit of this cytokine-targeted approach to atherosclerosis treatment was associated with reductions in IL-6 and hsCRP. The use of canakinumab for persistent AF after electrical cardioversion showed a lower incidence of AF recurrence, but the differences, compared with placebo, were not statistically significant.6
Colchicine has been shown to interfere with the NLRP3/IL-1β signalling pathway by inhibiting tubulin polymerisation. The Low Dose Colchicine (LoDoCo) trial, the Colchicine Cardiovascular Outcomes (COLCOT) trial and the LoDoCo 2 trial have demonstrated that treatment with colchicine for secondary prevention reduces atherosclerotic CVD. The Colchicine for the Prevention of the Postcardiotomy Syndrome (COPPS) AF substudy demonstrated a reduced incidence of paroxysmal AF after pericardiotomy. Subanalysis of a follow-up trial (COPPS-2) revealed a beneficial effect of colchicine for AF in patients undergoing cardiac surgery, but this was not statistically significant based on an intention-to-treat analysis.6 Colchicine and other anti-inflammatory agents that target the NLRP3 inflammasome pathway may be valuable tools in reducing the AF burden and preventing the progression of AF.
Immunoglobulin Free Light Chains: Novel Biomarkers of Inflammation in AF
Immunoglobulin comprises two identical heavy chains and two identical light chains, and provides a defence against all extracellular and some intracellular pathogens. In mammals, immunoglobulin light chain genes generally exist in two distinct isotypes, κ and λ, and are produced by B and plasma cells. Viral infection increases free light chain (FLC) levels in various body fluids, and the production of FLCs was considerably enhanced in a murine model of heart failure with encephalomyocarditis virus myocarditis.11 Furthermore, FLCs have protective effects in viral myocarditis, most likely through direct antiviral activity and an anti-inflammatory effect caused by the increased expression of IL-10.11
Markers of inflammation provide considerable insights into the underlying pathology of CVDs in patient assessment. Many risk factors of CVDs activate NF-κB (which regulates the transcription of immunoglobulin κ light chains in immunoglobulin-producing B and plasma cells) and the production of many inflammatory molecules, thus leading to inflammation. Therefore, FLCs were proposed as biomarkers of NF-κB activation and inflammation.15
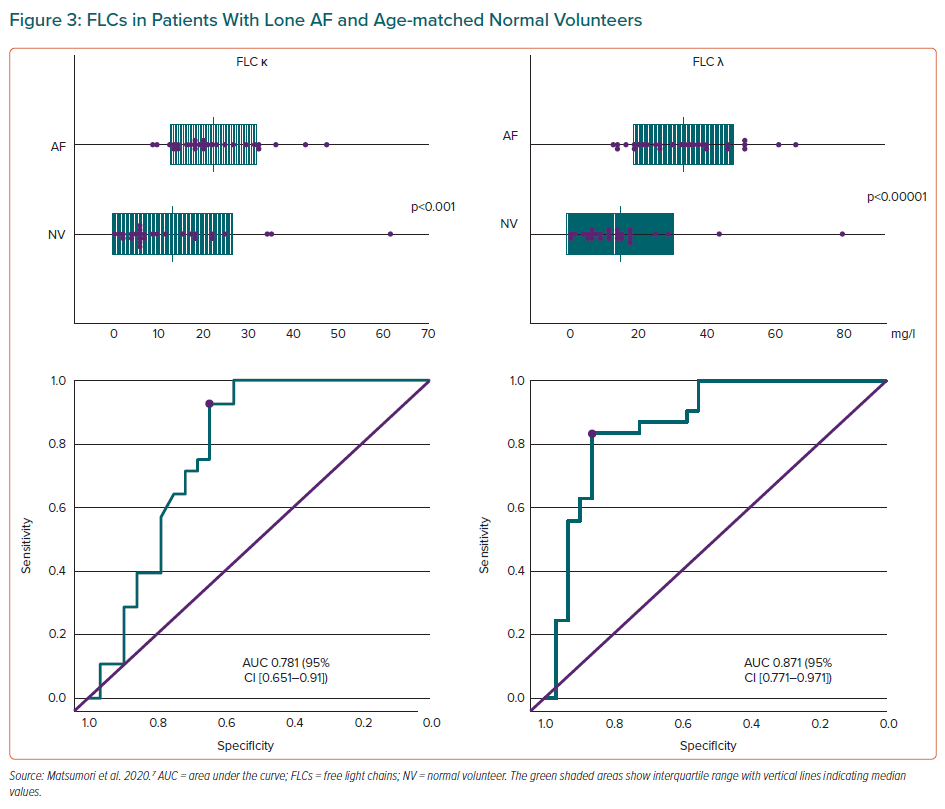
The hypothesis that there are differences in FLC concentrations between patients with lone AF and those who have heart failure when in sinus rhythm was recently tested.7 In that study, significantly higher concentrations of circulating FLC κ and λ were demonstrated in patients with lone AF compared with a healthy volunteer group. The area under the curve of the receiver operating characteristic curve showed that FLC κ and λ concentrations were helpful in differentiating patients with AF from healthy volunteers, and that the cut-off value of FLC κ or λ concentrations may help distinguish between the two groups (Figure 3).7
The findings of that previous study showed that the circulating concentrations of FLC λ were significantly higher in patients with heart failure with preserved and reduced left ventricular ejection fraction and myocarditis compared with controls, similar to the FLC λ findings in a recent study on heart failure patients in sinus rhythm.15
N-Terminal pro B-type natriuretic peptide (NT-proBNP) concentrations were elevated in patients with lone AF, but were not significantly correlated with FLC λ levels.7 Serum creatinine concentrations and the estimated glomerular filtration rate (eGFR) were not significantly correlated with FLC λ levels; however, NT-proBNP concentrations were significantly correlated with both creatinine and eGFR. Thus, the effects of creatinine or eGFR were less on FLC λ than NT-proBNP.
FLC κ and λ levels are increased in patients with AF, which suggests that clones of B lymphocytes and plasma cells that produce FLC κ and λ may be activated during AF, although the mechanism by which FLCs can cause AF remains to be clarified. The inflammation associated with FLCs may directly induce AF, or FLCs may change membrane fluidity, which, in turn, may alter ion channel function.
NF-κB and inflammasomes may be targets for new anti-inflammatory treatments for AF when FLC concentrations are elevated, and FLCs may be a surrogate endpoint of the efficacy of treatment of AF. Further studies are needed to clarify the role of FLCs in AF and whether FLCs may be included in screening tests for AF (Figure 1).
FLCs as biomarkers have the advantage of being stable and not changing after long-term storage.16 In a larger future study, we plan to examine potential confounders, such as types of AF, the severity of heart failure, gradations of renal function and other inflammatory processes and nutritional statuses.
We have shown that FLC κ and λ concentrations have diagnostic and prognostic value because of their correlation with the presence of AF. Furthermore, our work in COVID-19 myocarditis patients showed that FLC concentrations were more frequently elevated than hsCRP or IL-6 concentrations.11,17 Therefore, FLCs may be higher-quality biomarkers for both acute and chronic inflammation than hsCRP or IL-6. Of course, further studies are needed to assess this speculation.
We have shown increased circulating FLC λ concentrations and a decrease in the κ/λ ratio in chronic heart failure, myocarditis and hepatitis C virus infection.11,15 However, FLC κ concentrations increased in cases of acute viral disease, such as COVID-19.17 Furthermore, we recently showed that FLC λ concentrations increased and that FLCs are sensitive and specific diagnostic markers for type 2 diabetes, which is a strong risk factor for AF, suggesting that diabetes is an inflammatory disease.18,19
Although we still need to clarify the mechanisms underlying the different responses of B and plasma cells, FLC κ and λ may be differently regulated, because NF-κB may not exercise control over the production of FLC κ and λ in the same manner.16 The different responses of FLC κ and λ may be because antigens with limited epitopes tend to produce antibodies with restricted light chain usage, and antibodies with λ light chains have specificities that are different and complementary to κ light chain-containing antibodies.20
FLCs are suitable for the initial screening of CVDs and other inflammatory diseases in the general population. Secondary tests should be performed and followed up when abnormalities in FLCs are found, such as ECGs for AF and NT-proBNP/B-type natriuretic peptide for heart failure. Follow-up measurements of FLCs are useful for evaluating the effects of regimens such as diets, lifestyle changes or drug therapies.
Conclusion
AF is an inflammatory disease, and FLCs are novel inflammatory biomarkers of AF that may be useful for initial screening and for the assessment of various interventions for the management of AF.