Cardiogenic Shock Definition, Classification and Pathophysiology
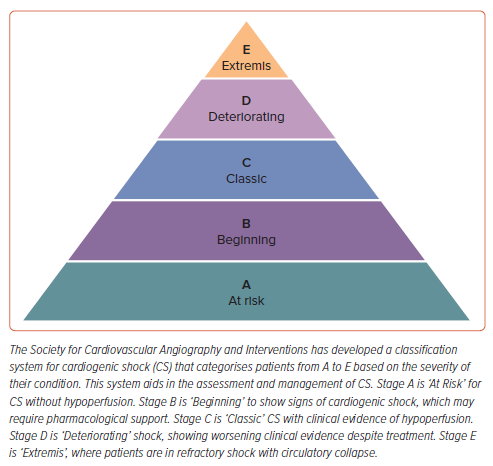
Cardiogenic shock (CS) is a critical condition in which the vital organs receive insufficient blood flow due to primary cardiac dysfunction, typically following acute MI, heart failure (HF) exacerbation, or myocarditis. It results from severe myocardial performance reduction, leading to decreased cardiac output, end-organ hypoperfusion, and hypoxia.1–3 The Society for Cardiovascular Angiography and Intervention (SCAI) classification system standardises the severity of CS and guides treatment strategies (Figure 1). The classification spans from stages A to E, ranging from patients at risk of developing CS to those in the most severe state. Stage A patients are ‘at risk’ of developing CS but do not exhibit signs of hypoperfusion or organ dysfunction. Stage B patients (‘beginning’) have begun to exhibit early signs of CS, such as hypotension (defined as a systolic blood pressure <90 mmHg) or tachycardia, without evidence of hypoperfusion or end-organ damage. Early intervention is crucial to prevent deterioration. Stage C (‘classic’) is characterised by hypotension with evidence of organ hypoperfusion, such as altered mental status, cool extremities, oliguria, or elevated lactate levels. Patients require pharmacological or mechanical support to maintain blood pressure and cardiac output. Stage D patients (‘deteriorating’) exhibit worsening signs of shock despite initial resuscitative efforts. They have persistent hypoperfusion and may require more aggressive support, such as high-dose vasopressors or mechanical circulatory support devices (e.g. intra-aortic balloon pump, ventricular assist devices). Finally, Stage E (‘Extremis’) is the most severe stage of CS, where patients are in refractory shock with critical hypoperfusion and multiple organ failure, even cardiac arrest, despite maximal interventions. Survival chances are significantly reduced, and treatment often involves advanced life support and consideration for emergent mechanical circulatory support or even cardiac transplantation.4–6
CS pathophysiology involves a vicious cycle of decreased myocardial contractility, systolic and diastolic ventricular dysfunction, systemic hypoperfusion, and subsequent further cardiac and multiorgan dysfunction.3,7,8 CS often results from acute MI, where a substantial portion of the myocardium is damaged, impairing the heart’s pumping ability. The reduced cardiac output leads to systemic hypoperfusion, initiating a series of compensatory mechanisms, including sympathetic nervous system activation, which increases heart rate and myocardial oxygen demand, and the renin–angiotensin–aldosterone system (RAAS) activation, which causes fluid retention and increases preload. However, these compensations may further strain the heart and exacerbate myocardial ischaemia. The resultant low blood pressure and decreased coronary artery perfusion pressure lead to worsening myocardial ischaemia, creating a vicious cycle that further diminishes cardiac output and leads to multisystem organ failure. The decreased perfusion results in anaerobic metabolism and lactic acidosis, which then contributes to the high mortality rate.8,9
Current standard treatments for CS focus on restoring haemodynamic stability using inotropic agents, vasopressors, mechanical circulatory support devices (e.g. intra-aortic balloon pump) and revascularisation if necessary. However, these treatments have limitations. Inotropics and vasopressors can increase myocardial oxygen demand and may lead to arrhythmias and increased mortality.10 Mechanical support devices are associated with significant complications, such as bleeding and thrombosis, and are not universally available.11
Overview of Levosimendan
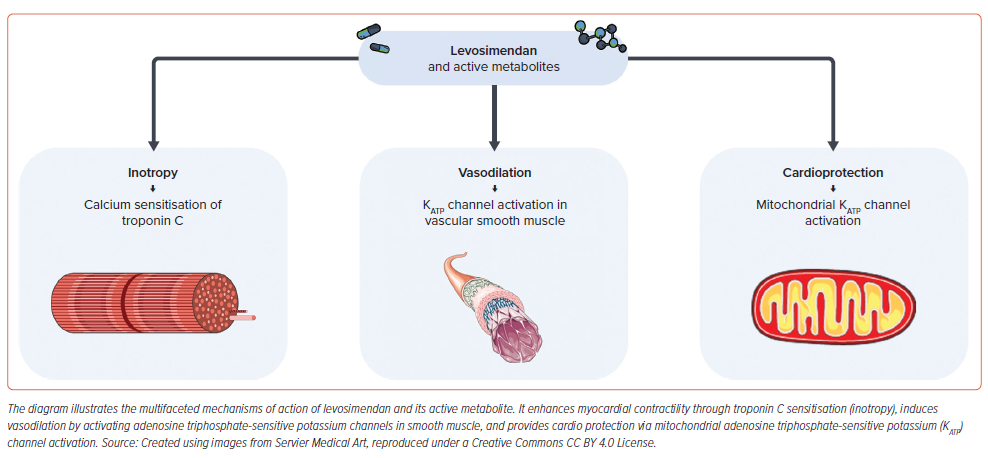
Levosimendan is a calcium sensitiser and adenosine triphosphate-dependent potassium (KATP) channel opener that improves myocardial contractility without significantly increasing intracellular calcium levels and oxygen demand. Unlike traditional inotropes, levosimendan enhances the sensitivity of cardiac troponin C (cTnC) to calcium, leading to a positive inotropic effect. Additionally, its vasodilatory action reduces both preload and afterload, potentially improving cardiac output and reducing myocardial workload. Levosimendan also exhibits anti-inflammatory and cardioprotective effects, offering advantages over conventional therapies (Figure 2).12,13
Furthermore, in vitro levosimendan is a highly selective phosphodiesterase 3 (PDE3) inhibitor.14,15 Nonetheless, this effect becomes significant only at higher doses than those used therapeutically, suggesting its primary mechanism of action is related to calcium sensitisation rather than PDE3 inhibition.16 Levosimendan is currently indicated for patients with acute decompensated heart failure (ADHF).17 In recent years, levosimendan has been a focus of numerous clinical trials.18 Studies have suggested its potential in CS, right ventricular failure, septic shock and other conditions that require inotropic support.19,20 This review aims to critically evaluate the efficacy and safety of levosimendan in CS management. Evidence is synthesised from recent studies, focusing on its impact on haemodynamic improvement, survival rates and clinical outcomes compared to standard treatments. This review also provides the role of levosimendan along with its practical use in the daily management of CS and identifies potential areas for future research.
Pharmacological Profile and Mechanisms of Action of Levosimendan
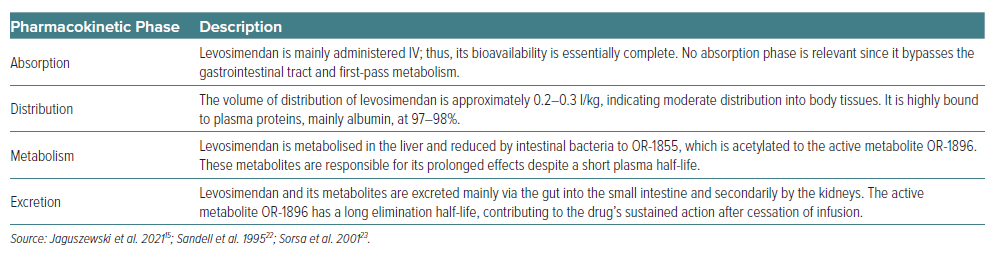
Levosimendan is a pharmacological agent with dual actions: it serves as a calcium sensitiser and opens KATP channels. Its pharmacokinetics and pharmacodynamics are characterised by a rapid onset and a prolonged effect, despite its relatively short plasma half-life (about 1 hour), owing to its active metabolites, OR-1896. The pharmacokinetics of levosimendan (summarised in Table 1) exhibit linearity within a therapeutic dosage range from 0.05 μg/kg/min to 0.2 μg/kg/min. Upon initiating IV infusion, therapeutic levels are typically attained around 1 hour later, with steady state achieved within 5 hours of continuous infusion. However, its effects are prolonged due to its metabolites, especially OR-1896, which possesses a significantly longer elimination half-life of 70–80 hours in patients with HF. The distribution volume for levosimendan is around 0.3 l/kg and exhibits a direct proportionality with a person’s body weight.
Levosimendan is predominantly bound to plasma proteins, with albumin being the main protein, accounting for 97–98% of its binding. The bioavailability and pharmacokinetics of levosimendan are affected by the formulation, with conventional tablets and capsules showing higher bioavailability compared to slow-release formulations.
Levosimendan is mainly excreted through the small intestine and the kidneys after being metabolised into active and inactive metabolites. The process involves its reduction by intestinal bacteria to an amino phenolpyridazinone metabolite (OR-1855), which is further metabolised through acetylation to the N-acetylated conjugate OR-1896. The active metabolite OR-1896, which contributes to the prolonged haemodynamic effects of levosimendan, is formed slowly, with its maximum concentrations typically observed around 2 days after stopping a 24-hour infusion of levosimendan. While the primary route of levosimendan metabolism involves gut bacteria, the liver also plays a role. However, specific details on the involvement of cytochrome P450 (CYP) enzymes are less clear.14,21,22 Following a 24-hour infusion of levosimendan, the pharmacodynamic effect persists for a minimum of 1 week.14
Pharmacodynamically, levosimendan enhances myocardial contractility through calcium sensitisation of cTnC without increasing intracellular calcium concentration, avoiding excessive oxygen consumption. Initially, levosimendan binds to cTnC in a calcium-dependent manner. The binding occurs specifically at the N-terminal domain of cTnC, which is crucial for calcium-induced conformational changes that allow actin-myosin cross-bridge cycling and thus muscle contraction. By stabilising the calcium-bound state of cTnC, levosimendan enhances the sensitivity of the heart muscle to calcium. The interaction between levosimendan and cTnC stabilises the complex of cTnC with calcium, prolonging the time calcium remains bound to cTnC. This action enhances the contractile process by making more effective use of the calcium that enters the myocardial cells during each cardiac cycle, leading to stronger contractions without the need for additional calcium. By increasing the calcium sensitivity of the heart muscle, levosimendan effectively increases the force of cardiac muscle contraction under conditions where calcium concentration would not normally be sufficient to produce such a strong response. This is particularly beneficial in heart failure, where the heart’s ability to contract effectively is compromised. Unlike other inotropic agents that increase intracellular calcium levels, possibly leading to detrimental effects such as arrhythmias and increased oxygen demand, levosimendan achieves its positive inotropic effect without increasing intracellular calcium concentrations. This results in an improved contractile function with less risk of calcium overload and subsequent cardiac dysfunction. Despite its effects on systolic function, levosimendan does not impair diastolic relaxation as the decay of calcium transient takes place prior to the peak contraction and the beginning of relaxation. The mechanism ensures that while contractions are stronger, the relaxation phase of the cardiac cycle is not negatively affected, maintaining overall cardiac efficiency and function.23–25
Levosimendan also induces vasodilation, leading to reduced preload and afterload. This effect is attained through several mechanisms, primarily involving the opening of KATP channels in vascular smooth muscle cells. When these channels are opened, potassium ions flow out of the cells, leading to hyperpolarisation of the cell membrane. This hyperpolarisation prevents the opening of voltage-dependent calcium channels, thereby reducing intracellular calcium concentrations. Since calcium ions are crucial for smooth muscle contraction, their reduced availability leads to the relaxation of the smooth muscle and vasodilation. There is some evidence to suggest that levosimendan may also have beneficial effects on endothelial function, potentially leading to enhanced release of endothelium-derived relaxing factors, such as nitric oxide (NO) through p38 mitogen-activated protein kinase (MAPK), ERK and Akt pathways. Although this is not the primary mechanism of vasodilation induced by levosimendan, improved endothelial function can complement the overall vasodilatory effect of the drug in the cardiovascular system.26–28
Levosimendan has been shown to have cardioprotective effects by activating mitochondrial KATP channels, contributing to its efficacy in treating heart failure and cardiogenic shock.27,28 This protection mechanism involves several cellular and molecular pathways that collectively help to preserve mitochondrial function, reduce oxidative stress, and enhance cell survival under ischaemic conditions. Activation of mitochondrial KATP channels leads to potassium influx into the mitochondria, which helps maintain the mitochondrial membrane potential. This stabilisation is crucial for preserving mitochondrial function, including ATP production, especially during the reperfusion phase following ischaemic events which potentially occur during CS. In this case, maintaining ATP levels is vital for cell survival and function, as it ensures the energy supply for critical cellular processes.29
Furthermore, by stabilising the mitochondrial membrane potential, the activation of mitochondrial KATP channels also helps prevent calcium overload in mitochondria. Excessive calcium in mitochondria during reperfusion can trigger the opening of the mitochondrial permeability transition pore (mPTP), leading to cell death through necrosis or apoptosis. Thus, preventing calcium overload is crucial for cell survival.30 Activation of mitochondrial KATP channels has also been associated with an increase in the production of reactive oxygen species (ROS) at a level that does not cause damage but is sufficient to trigger protective signalling pathways. This mild increase in ROS can enhance antioxidant defences, making the heart more resistant to oxidative stress.31 Finally, activation of mitochondrial KATP channels can prevent the release of cytochrome C from mitochondria, a key event in the initiation of apoptosis. By inhibiting this release, the channels help prevent apoptosis, contributing to cell survival during and after ischaemic events.32
Distinct Pharmacological Profile of Levosimendan Shapes its Clinical Features
The distinct pharmacological profile of levosimendan significantly impacts haemodynamic parameters critical for managing HF and CS. Its dual mechanism of action sets it apart from traditional inotropic agents. Levosimendan’s positive inotropic effect is primarily attributed to its action on cTnC, increasing calcium sensitivity and, consequently, myocardial contractility without elevating intracellular calcium levels. This mechanism facilitates an increase in stroke volume and cardiac output, essential for reversing the low-output state observed in HF and CS patients.10 Studies have consistently shown that levosimendan administration leads to a significant improvement in these parameters, contributing to enhanced peripheral and organ perfusion.12 In addition to its inotropic effects, levosimendan exerts vasodilatory actions on both arterial and venous vessels. This reduces systemic vascular resistance (SVR), lowering afterload and further aiding in the improvement of cardiac output.33 The reduction in SVR also contributes to decreased myocardial oxygen demand, a vital aspect in managing patients with compromised cardiac function.13
The haemodynamic improvements induced by levosimendan are not just theoretical advantages but translate into meaningful clinical benefits. A previous study evaluated the haemodynamic effects of levosimendan on left and right ventricular function in patients with intractable CS following MI. The treatment was associated with a significant increase in cardiac index and enhanced right ventricular cardiac power index.34 In acute decompensated HF, treatment with dobutamine or levosimendan led to significant reductions in biomarkers like b-type natriuretic peptide (BNP), N-terminal pro BNP/ST2 (NT-proBNP/ST2), and an increase in glomerular filtration rate (GFR).35,36 Interestingly, improvement in haemodynamic parameters was only seen in those treated with levosimendan.35 By enhancing cardiac output and reducing afterload, levosimendan addresses the fundamental pathophysiological aspects of HF and CS, potentially leading to improved outcomes and reduced mortality.
Clinical Efficacy of Levosimendan versus Other Inotropic Agents
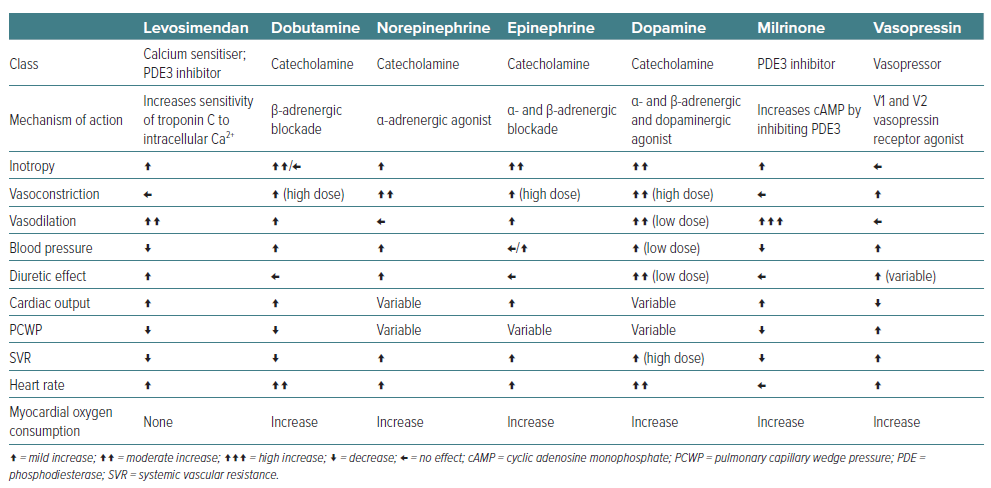
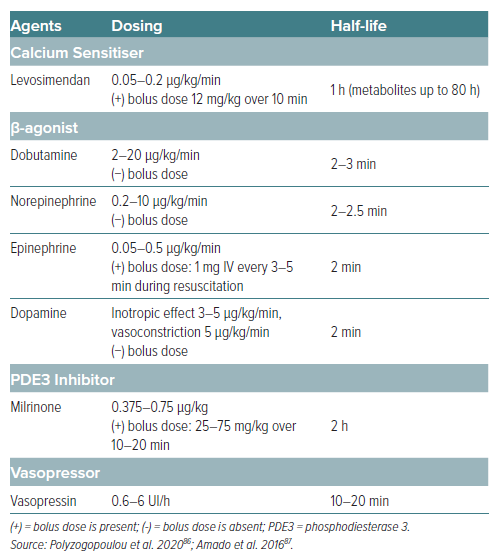
The management of CS often necessitates the use of inotropic agents to improve cardiac output and organ perfusion. Levosimendan has emerged as a novel agent with distinct advantages over traditional inotropes such as dobutamine, enoximone, dopamine, and norepinephrine. Levosimendan’s mechanism of action offers haemodynamic benefits without significantly increasing myocardial oxygen demand. This contrasts with the mechanisms of dobutamine and dopamine, which increase intracellular cAMP and may lead to heightened myocardial oxygen consumption (Table 2) and arrhythmic risks.10
In a study comparing levosimendan to enoximone in refractory CS complicating acute MI, it was found that levosimendan contributed to improved survival compared to enoximone (69% versus 37%, p=0.023), with levosimendan inducing higher cardiac index and lower cumulative values for catecholamines at 72 hours.37 Likewise, when compared to dobutamine, levosimendan improved cardiac power output better in acute MI patients who developed CS following percutaneous coronary intervention (PCI).38 Interestingly, long-term analysis on the same patients demonstrated no significant differences in long-term survival between both groups (p=0.24).39 The timeframe of outcome evaluation must be considered cautiously. The study revealed the superiority of levosimendan over dobutamine in improving haemodynamic function within 24 hours; however, 180-day analysis did not show the difference. Nevertheless, it is worth mentioning that the sample size was considered small and there was a difference in baseline coronary artery disease characteristics between both therapy groups.38,39
A critical analysis of survival rates in various studies reveals that levosimendan is associated with improved outcomes compared to dobutamine, particularly in patients with severe low-output HF. The LIDO study demonstrated a significant reduction (HR 0.57; 95% CI [0.34–0.95]; p=0.029) in mortality at 180 days in patients treated with levosimendan compared to those receiving dobutamine, highlighting a clear survival advantage of levosimendan over dobutamine in patients with severe HF.40 The SURVIVE trial further supports these findings, suggesting that levosimendan may lead to better survival rates compared to dobutamine.41 Moreover, levosimendan has shown a better safety profile, with fewer instances of tachyarrhythmias and ischaemia than dobutamine and dopamine (i.e. catecholamine-based inotropes), and unlike norepinephrine, it does not significantly increase afterload due to its vasodilatory effects.42,43 The vasodilatory effects of levosimendan also offer therapeutic advantages by improving renal perfusion and diuresis, a crucial aspect in managing CS patients with concomitant acute kidney injury.44,45
Finally, clinical trials and meta-analyses have also suggested a survival benefit associated with levosimendan compared to other inotropes, particularly in patients with severe HF and those undergoing cardiac surgery.10,40,46 The improved survival rates can be attributed to the unique haemodynamic effects of levosimendan, including enhanced cardiac output and reduced preload and afterload, without the detrimental increase in myocardial oxygen consumption observed with other inotropes. Haemodynamically, levosimendan also leads to reductions in pulmonary capillary wedge pressure (Table 2), which are critical in the management of CS. These effects are achieved with a lower risk of tachycardia and arrhythmias compared to dobutamine and dopamine. The vasodilatory effect of levosimendan also distinguishes it from norepinephrine, which may increase afterload and worsen cardiac function in certain settings.47 In terms of safety, levosimendan has a favourable side-effect profile, particularly concerning arrhythmic risk and myocardial ischaemia. While dobutamine and dopamine can increase the risk of arrhythmias and norepinephrine can lead to significant vasoconstriction, levosimendan provides a balanced approach to inotropic support with fewer adverse effects.40,48–50
Clinical Usage in Various Specific Settings
Levosimendan has shown promising outcomes across a spectrum of cardiovascular emergencies and chronic conditions, extending its utility beyond the management of acute HF and CS. In the setting of AMI complicated by HF, levosimendan offers haemodynamic support by improving myocardial contractility and reducing preload and afterload, potentially mitigating the progression to CS. Studies have demonstrated the ability of levosimendan to improve outcomes in AMI patients, particularly when conventional inotropic support is insufficient or associated with adverse effects.10,51 Its cardioprotective effects, coupled with the ability to improve coronary flow and reduce MI size, have been shown by some animal studies. In dogs, levosimendan could reduce left ventricular (LV) infarction size from 24% ± 2% to 11% ± 2%.52 A study in an experimental pig model also demonstrated significant difference (p=0.03) in LV infarction size between the levosimendan group (12% ± 13%) and the control group (27% ± 15%).27
Several studies evaluated the effect of levosimendan on weaning patients from veno-arterial extracorporeal membrane oxygenation (VA-ECMO), typically used in severe cardiopulmonary failures like CS and during extracorporeal cardiopulmonary resuscitation (eCPR) following cardiac arrest. The analysis found that the levosimendan group had a significantly higher success rate of weaning from ECMO, with a reduced mortality rate at 28 or 30 days, although patients on levosimendan had longer durations of ECMO support.53–56 Likewise, a study investigating the adjuvant efficacy of levosimendan in patients with temporary ventricular-assisted devices (VADs) for CS demonstrated that immediate administration of levosimendan after VAD implantation significantly improved mean arterial pressure, reduced lactate levels, and enhanced arterial oxygen partial pressure to fractional inspired oxygen ratio. Furthermore, patients treated with levosimendan exhibited improved systolic function and pulmonary artery pressure, leading to a higher weaning rate and lower mortality rate at the 6-month follow-up compared to the control group.57
Nevertheless, an observational cohort study conducted on 200 adult patients with refractory CS undergoing VA-ECMO revealed that despite a longer ECMO duration in the levosimendan group, there was no significant difference in the rate of weaning failure, suggesting that levosimendan did not improve VA-ECMO weaning success in this patient population.58
On the other hand, traditional inotropics, such as dobutamine, have been broadly used in clinical practice; however, studies have found an increase in mortality due to myocardial ischaemia and arrhythmias.43 To the best of our knowledge, there is no study directly comparing levosimendan with other inotropics such as dobutamine in terms of weaning from VA-ECMO.
Peripartum cardiomyopathy (PPCM) and takotsubo cardiomyopathy (TCM) represent specific clinical scenarios. Experimental studies have found that catecholamines, such as dobutamine, should be avoided in PPCM due to their teratogenicity.59 In this case, the unique action profile of levosimendan can be particularly beneficial. In PPCM, levosimendan has been shown to improve cardiac function and facilitate recovery of LV function.60 The ability of levosimendan to improve myocardial contractility without significantly increasing heart rate and oxygen consumption makes it an appealing option for PPCM patients, who may be vulnerable to the side-effects of traditional inotropes. Studies have shown that levosimendan can facilitate haemodynamic improvement and potentially aid in the recovery of ventricular function in PPCM.61 Similarly, in TCM, which involves transient LV apical ballooning typically triggered by emotional or physical stress, the usage of inotropic such as dobutamine could trigger vasospasm or catecholamine surge, which will potentially lead to adverse outcome or even induce the TCM itself.62,63
A study comparing levosimendan and dobutamine in the management of TCM found that the recovery of LV systolic function only occurred in levosimendan group.64 Levosimendan can also provide symptomatic relief and haemodynamic stabilisation, addressing the acute systolic dysfunction.13,65 Patients undergoing cardiac surgery, particularly those with pre-existing LV dysfunction, are at increased risk of low cardiac output syndrome (LCOS) postoperatively. Levosimendan, administered preoperatively or intraoperatively, has been associated with improved postoperative cardiac function, reduced need for mechanical circulatory support, decreased incidence of LCOS, and even decreased mortality, compared to placebo, dobutamine or milrinone.10,66 When compared to dobutamine, levosimendan also shows better improvement in cardiac index, systolic volume index, NT-proBNP, and kidney function.67 Its ability to provide inotropic support without significantly increasing myocardial oxygen demand makes it an attractive option in this vulnerable patient population.
Haemodynamic improvement also extends beyond acute management, suggesting potential long-term benefits of levosimendan in HF management. The ability of levosimendan to improve cardiac function while minimising adverse effects also presents a compelling case for its use as a bridging therapy in patients awaiting more definitive interventions, such as mechanical circulatory support or transplantation. In addition, its vasodilatory properties can ameliorate pulmonary hypertension, a common complication in right HF and chronic HF conditions, offering symptomatic relief and improving clinical outcomes.12 The broad applicability of levosimendan across various clinical scenarios underscores its potential as a versatile therapeutic agent in cardiovascular medicine. Its efficacy in improving haemodynamic parameters and survival, coupled with a favourable safety profile, supports its use in carefully selected patients. Further research and randomised clinical trials are warranted to optimise its use, define its role in treatment protocols, and explore its benefits in other cardiovascular conditions.
Safety and Adverse Effects of Levosimendan
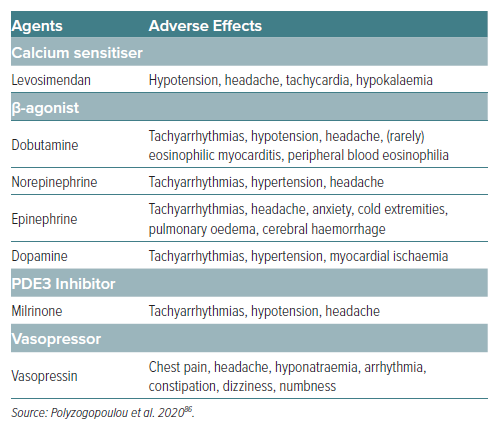
While levosimendan has shown significant promise in treating HF, CS, and other cardiovascular conditions, understanding its safety profile and potential adverse effects is crucial for optimising patient outcomes. Levosimendan is generally well-tolerated, with the most common adverse effects being hypotension and headache.
A meta-analysis of 25 randomised controlled trials found that the risk of headache or migraine and hypotension was higher at levosimendan compared to placebo or dobutamine.68 These effects are attributed to its vasodilatory action, which, while beneficial for reducing preload and afterload, can lead to symptomatic hypotension in some patients.12 Other reported side-effects include dizziness, nausea and an increase in heart rate, which are typically mild and transient. In addition, levosimendan administration also led to a significantly greater reduction in mean haemoglobin values compared to dobutamine.69 Serious adverse effects of levosimendan are rare, but may include severe hypotension and cardiac arrhythmias, including AF. These effects are more likely to occur in patients with underlying severe aortic stenosis or those receiving concurrent treatment with other vasodilators or negative chronotropic agents. Ventricular arrhythmias have been reported but are less common than with β-adrenergic agonists such as dobutamine.13 However, conflicting evidence exists concerning ventricular tachycardia (VT) events. The REVIVE study demonstrated a higher incidence of VT with levosimendan versus placebo, while the SURVIVE Study reported similar rates.17
When compared with other inotropic agents (Table 3), levosimendan exhibits a unique safety profile. Its mechanism of action, which does not rely on increasing intracellular calcium levels, may account for the lower incidence of arrhythmogenic effects compared to agents such as dobutamine or milrinone. In an in vitro study investigating the effects of levosimendan, milrinone and isoprenaline on human-induced pluripotent stem cell (hiPSC)-derived cardiomyocytes at hypothermic temperatures, milrinone and isoprenaline were found to significantly increase the risk of ventricular arrhythmias by increasing action potential triangulation. Remarkably, levosimendan did not increase triangulation and maintained contractile properties even at temperatures as low as 26°C. Therefore, levosimendan may be a safer option for inotropic treatment in hypothermic patients.70 Studies have also indicated that the risk of adverse renal effects is lower with levosimendan than with other inotropes, which is particularly relevant in HF patients, where renal function is a critical determinant of outcome.10
Furthermore, levosimendan has been associated with a reduced need for mechanical circulatory support compared to dobutamine, suggesting its role in more effectively stabilising critically ill patients with fewer complications.12 Overall, levosimendan offers a comparatively safe alternative to traditional inotropic agents, with a side-effect profile that supports its use in specific patient populations. While its beneficial haemodynamic effects are clear, careful patient selection and monitoring are essential to mitigate the risks of hypotension and other adverse effects. Ongoing research and post-marketing surveillance will further clarify the safety of levosimendan, especially in comparison with other cardiovascular therapies.
Application of Levosimendan in Daily Clinical Practice
Considering the safety profile of levosimendan and its hypotension risk, an initial high-dose bolus of levosimendan alone is generally not recommended in patients with hypovolemia or low blood pressure at the beginning. It is only used once the immediate effect has been observed or the systolic blood pressure is adequate.71 Therefore, levosimendan alone is only used when SBP is >90 mmHg. Otherwise, a combination with other inotropic and vasopressor agents is most recommended.56
Several studies have demonstrated the clinical benefit of these combinations. The combination of vasoactive catecholamines and levosimendan has shown significant increase in cardiac index and decrease in systemic vascular resistance in critically ill patients with cardiogenic shock.33 Combining levosimendan with inotropes such as dobutamine is also promising. The CTnC calcium sensitisation property in levosimendan and calcium intracellular concentration enhancement in dobutamine would be expected to generate great potentiation in their efficacy.72 The first study combining levosimendan and dobutamine for treating patients with severe cardiac failure has shown significant increase in cardiac index and significant decrease in pulmonary capillary wedge pressure within 24 hours of administration.73 Longer follow-up even revealed prolonged survival rates in patients with combination, as compared to dobutamine alone.74 Another randomised study in 89 patients with acute decompensated heart failure also demonstrated the superiority of the levosimendan-dobutamine combination group over the dobutamine-only group in terms of preventing MACE outcomes.75
Dosing Considerations and Monitoring Requirements
The dosing of levosimendan must be tailored to the individual patient’s clinical status and underlying cardiac function. Typically, a loading dose is followed by a continuous infusion, adjusted based on haemodynamic response and tolerance. Initiating therapy involves administering a loading dose (which is presently not advised for severely hypotensive patients) ranging from 6 μg/kg to 12 μg/kg over 10 minutes, followed by a continuous infusion at a rate of 0.1 μg/kg/min. Adjustments to the continuous infusion rate can be made by decreasing it to 0.05 μg/kg/min, pausing it if the response is deemed too strong, or raising it to 0.2 μg/kg/min if the initial dose is well tolerated and a stronger haemodynamic effect is desired (Table 4).14 For PPCM patients and those undergoing cardiac surgery, careful dosing is crucial to avoid hypotension or excessive vasodilation, with some guidelines suggesting lower initial doses or omitting the loading dose in certain scenarios.12 Close monitoring of haemodynamic parameters, including blood pressure, heart rate and cardiac output, is essential during levosimendan therapy. In addition, electrolytes and renal function should be monitored due to the potential for hypotension and its effects on renal perfusion. For patients with PPCM, monitoring extends to assessing ventricular recovery through imaging and biomarkers. In the perioperative cardiac surgery setting, vigilance for signs of LCOS and arrhythmias is paramount, guiding the adjustment of levosimendan therapy and the management of fluid status and concomitant medications.
Future Perspectives
Recent research and ongoing trials are paving the way for the application of levosimendan in a broader array of cardiovascular conditions and patient populations. Emerging evidence suggests that levosimendan may benefit patients with right ventricular failure, septic shock, and advanced chronic HF, where conventional inotropic support options are limited or associated with significant adverse effects.76–81 Several clinical trials are currently underway to explore new indications for levosimendan. For instance, the LION-HEART study is evaluating the long-term use of intermittent levosimendan infusions in patients with advanced HF, aiming to assess its impact on hospitalisation rates and survival.74 Another area of active research is the use of levosimendan in paediatric populations, particularly in children with HF or undergoing cardiac surgeries, where its safety and efficacy parameters are being rigorously tested.82–84 Research is also focusing on identifying specific patient groups that may derive the most benefit from levosimendan therapy. This includes patients with HF with preserved ejection fraction (HFpEF), where treatment options are currently limited, and patients with acute decompensation due to pulmonary embolism or acute renal failure. In the ZSF1 obese rat model of HfpEF, chronic treatment with the inodilator levosimendan improved effort tolerance, enhanced LV relaxation and diastolic compliance, and reduced hypertrophy and interstitial fibrosis. Acutely, levosimendan was found to decrease systemic arterial pressures and increase cardiac index. The results of this study suggest that levosimendan may be a promising therapeutic agent for HfpEF and warrants further clinical trials in a broad patient population.85 Next, the potential for levosimendan to improve renal perfusion and function presents an intriguing avenue for reducing the morbidity associated with acute kidney injury in HF patients. Also, technological advancements in drug delivery and monitoring are expected to enhance the clinical utility of levosimendan. Novel drug delivery systems, such as subcutaneous or oral formulations are under investigation, which could facilitate outpatient management of chronic HF patients and improve adherence to therapy. Additionally, integration with advanced haemodynamic monitoring techniques, along with the optimal dose and administration, needs to be explored further to maximise therapeutic benefits.
Conclusion
Levosimendan has emerged as a pivotal agent in the management of CS, offering significant haemodynamic benefits that extend beyond the limitations of traditional inotropic and vasopressor therapies. Its unique mechanism of action, which enhances myocardial contractility via calcium sensitisation and induces vasodilation through KATP channel opening, provides a balanced approach to improving cardiac output and reducing systemic vascular resistance without the detrimental increase in myocardial oxygen consumption often associated with other inotropes. Comparative studies have underscored the superior efficacy of levosimendan in improving survival rates, particularly in patients with severe HF and those undergoing high-risk cardiac surgeries. Its ability to improve haemodynamic parameters while exhibiting a favourable safety profile, especially in terms of reducing the incidence of arrhythmias and myocardial ischaemia, positions levosimendan as a valuable tool in the critical care setting. The integration of levosimendan in clinical practice has been supported by evidence demonstrating its benefits in specific patient populations, including those with acute MI, acute HF, PPCM and takotsubo cardiomyopathy, among others. Ongoing research and clinical trials are expected to further delineate its role across a broader spectrum of cardiovascular conditions, potentially expanding its indications and solidifying its place in treatment algorithms. As our understanding of the clinical utility of levosimendan deepens, its continued evaluation and integration into therapeutic strategies are essential for enhancing patient care and outcomes in the complex landscape of CS management.