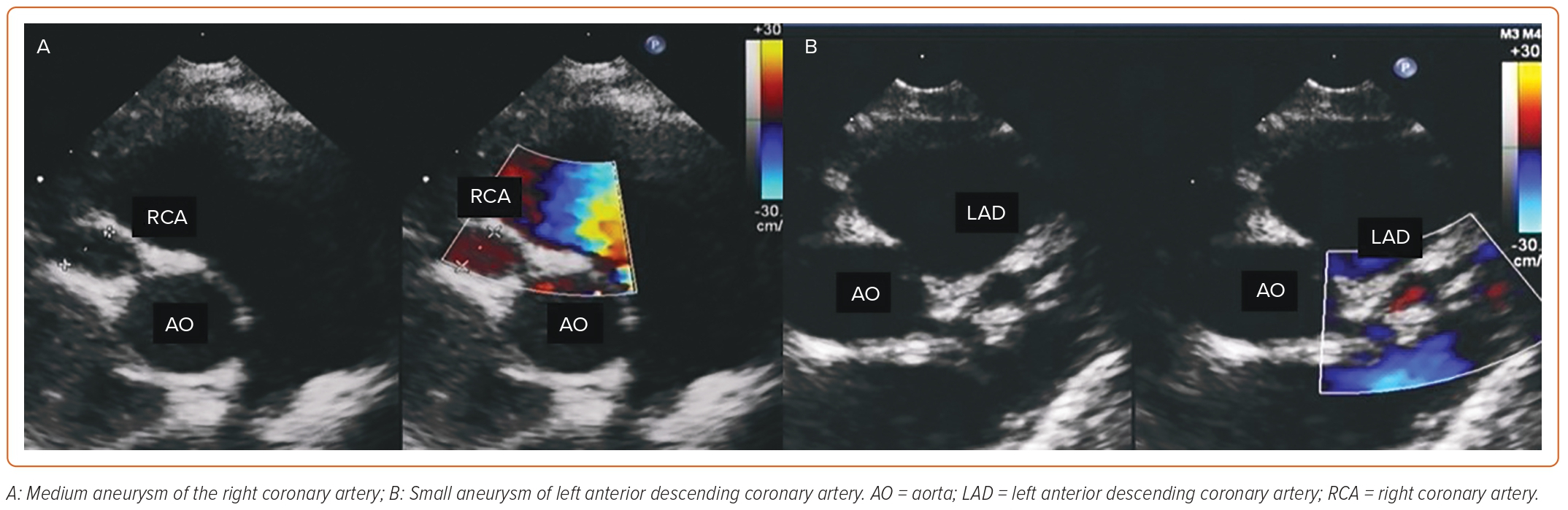
Kawasaki disease (KD) is an inflammatory disorder that can affect the entire cardiovascular system, especially the coronary arteries, in infants (Figure 1).1 Its cause is still unknown, but viruses and other indirect agents may play a role in the onset of KD.2 This may be supported by the seasonal increase in KD cases worldwide, as well as the detection of several variants of classical KD during the most recent COVID-19 pandemic. KD seems to be more prevalent in genetically predisposed individuals, being more common in Asia (322/100,000) than in Europe and the US (4–25/100,000).1
Apart from rheumatic fever in developing countries, KD is the leading cause of acquired heart disease in the world. Even though the incidence of KD is high, the use of IV immunoglobulin (IVIG) has changed the natural course of the disease. Interestingly, up to 6% of treated patients may develop coronary artery aneurysms (CAA), even after IVIG use.3 A recent multicentre study in Latin America revealed that up to 21% of the KD patients had CAA detected during their follow-up.4 Remarkably, prior to the use of IVIG, the rate of CAA regression was 55% in the first 2 years of KD, whereas it is currently 75% with the use of IVIG.5,6 Additionally, only 19% of patients with giant CAA (z-score ≥10) at the time of diagnosis experienced regression, and up to 5% of these patients may experience severe cardiovascular events.6
Although giant CAA are kept in surveillance, patients with self-limited coronary dilatation or small and medium aneurysms are discharged after a maximum of 5 years of follow-up; there is no strong evidence that they are free of cardiovascular events.5 Indeed, there is a description of coronary artery wall impairment in an autopsy of a patient who died for reasons other than previous KD, where serial echocardiograms diagnosed ‘intact coronaries’.7 The inflammatory process damages the endothelium of the artery, rendering it permanently dysfunctional. Particularly after resolution of the giant CAA, where layering thrombi and luminal myofibroblastic proliferation are observed, the risk of ischaemic events is enhanced.8 Therefore, we aimed to cover the most important aspects of KD pathophysiology, treatment and follow-up.
Pathophysiology of Coronary Artery Lesions in KD
Acute and Subacute Stages
In the first 2 weeks after fever onset (acute phase), a complex interaction of cellular and humoral immune responses starts, beginning with neutrophils activation and the release of interleukins-1 and 6, and tumour necrosis factor. This appears to be followed by macrophages and cellular infiltration in the intima layer of coronary arteries and, consequently, vessel wall disruption, resulting in the formation of aneurysms, which are more prevalent in the proximal segments of the artery.8
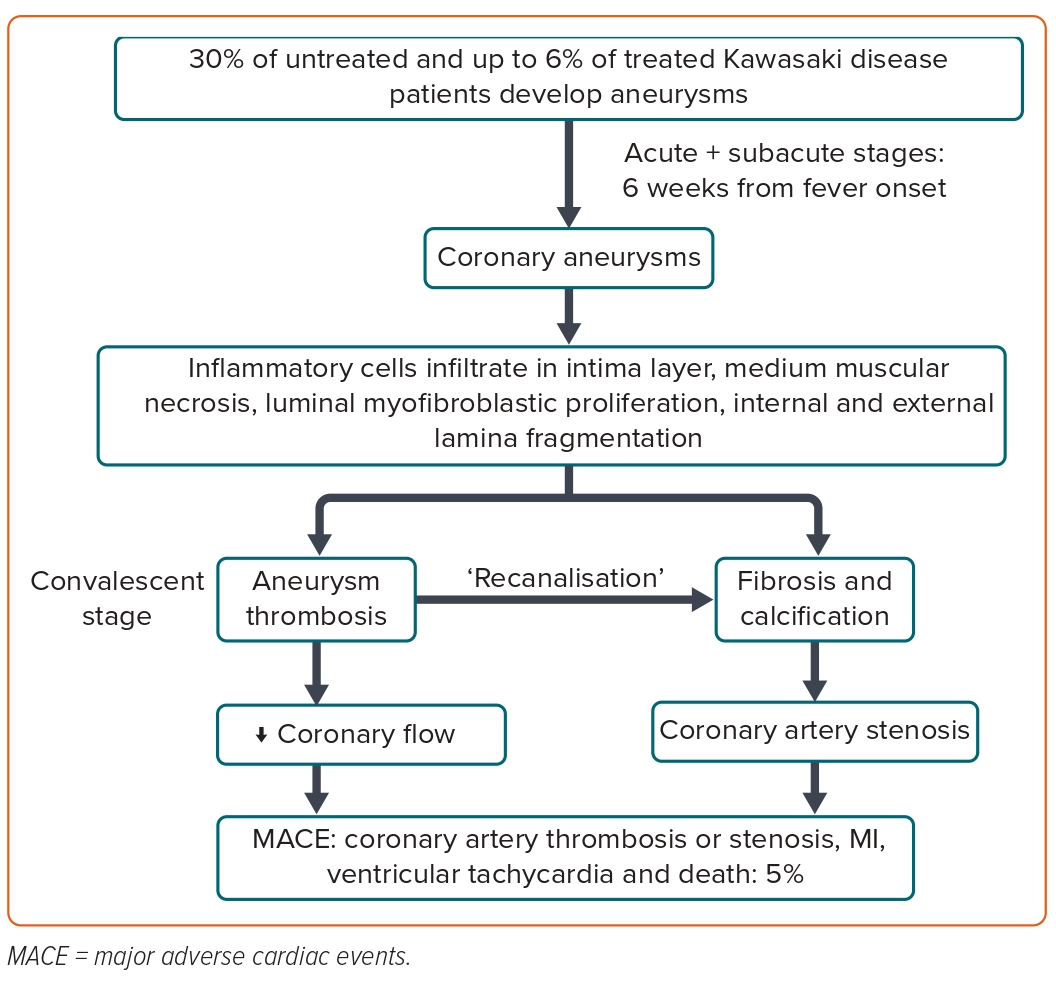
Isolated distal coronary involvement is exceedingly rare. In the subacute stage (2–6 weeks), luminal myofibroblast proliferation occurs alongside progressive thickening of the intima and arterial obstruction.9 Currently, it is believed that luminal myofibroblast proliferation is an active proliferative process that plays a crucial role in arterial remodelling in KD (Figure 2).1 A recent validation of a risk model for predicting coronary aneurysms in the North American population included four variables: maximum z-score of the LAD or right coronary artery (RCA) ≥2, age at fever onset <6 months, C-reactive protein ≥13 mg/dl and Asian ancestry.10 Two points are attributed to coronary artery abnormalities, while 1 point is attributed to the remaining variables. High-risk patients score ≥3 points.10
Convalescent Stage
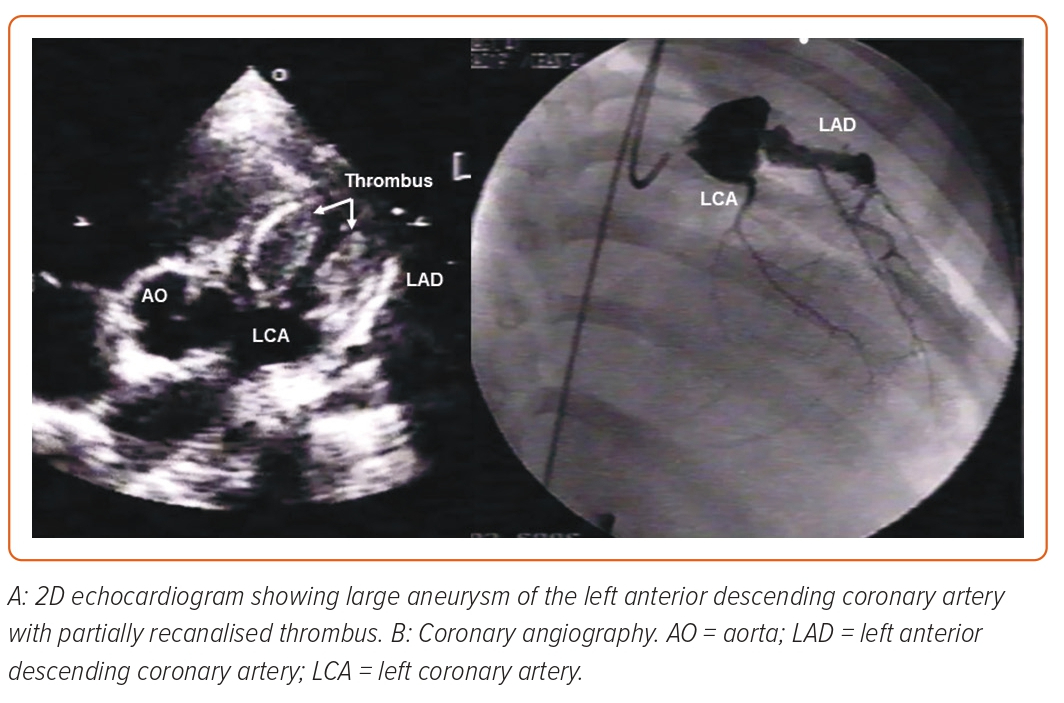
After the acute and subacute stages of KD, chronic vasculitis, characterised by an infiltration of plasma cells, lymphocytes and eosinophils, can continue for years in a small proportion of patients.8 It is during this phase that progressive thickening of the intima can lead to left main stenosis, particularly at the aneurysm-normal artery junction. Commonly observed in the arterial wall of aneurysms, calcification may be induced by osteoblasts derived from the medial smooth muscle cells.9 The enhanced platelet aggregation and coagulation, as well as the suppression of the fibrinolytic system, facilitate the formation of thrombus in massive coronary aneurysms after KD, which is one of the most serious complications (Figure 2).11
Notably, the giant aneurysms show myointimal proliferation, associated with disrupted internal elastic lamina, which, along with medial smooth muscle cell necrosis followed by fibrosis, decreases the likelihood of regeneration.12 According to Tsuda et al., large or giant aneurysms (z-score ≥10) are associated with up to a 50% risk of thrombotic coronary occlusion, progressive stenosis requiring revascularisation or acute coronary syndrome in 30 years of follow-up (Figure 3 and Supplementary Material Figure 1).12
In a recent multicentre study of 1,006 patients with KD aged <19 years who underwent coronary angiography, the 10-year event-free survival rate revealed that the most significant findings related to major coronary events were large aneurysms, male sex and resistance to IVIG therapy.13
As demonstrated in a study where flow-mediated dilatation, which is an ultrasound technique to measure arterial responsiveness to reactive hyperaemia, showed reduced flow dilatation and coronary reserve in KD with a history of transiently dilated coronary arteries, we should all consider the possibility that coronary involvement extends beyond dilatations and stenosis to include potentially functional endothelium impairment.14,15
Other Potential Cardiovascular Complications of KD
In the early phases of KD, myocarditis is also possible; left ventricular (LV) dysfunction is typically mild and improves with treatment. Extremely uncommon, critical cardiogenic shock appears to be associated with IVIG resistance and coronary artery abnormalities.5 In a subset of KD patients, diffuse myocarditis followed by fibrosis may lead to systolic or diastolic dysfunction (Supplementary Material Figure 2). In fact, mid-term follow-up studies have demonstrated subclinical myocardial involvement using strain analysis derived from speckle tracking. In addition, ventricular arrhythmias with a late onset and congestive heart failure may develop.16 It is unknown whether diffuse fibrosis is the result of ischaemic injury from microinfarcts or inflammatory cardiomyocyte injury.5
Pericardial effusion and valvulitis are commonly described in the acute phase, both of which are considered transient in the majority of patients. Ascending aorta dilatation, as well as aneurysms of axillary and iliac/femoral arteries, have been described.5
Evaluation of Cardiac Impairment in KD
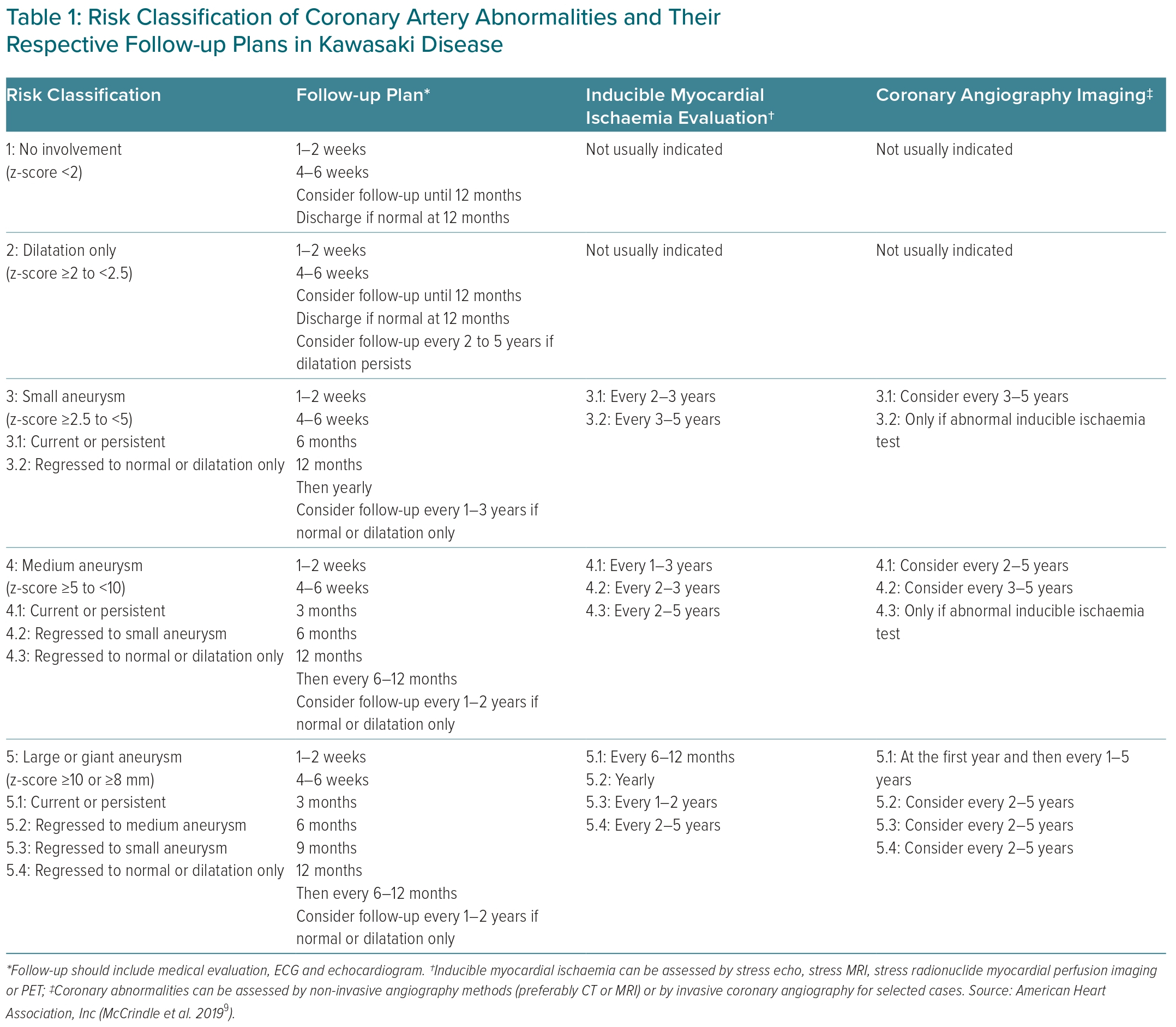
KD follow-up and diagnostic testing protocols are based on cardiovascular risk stratification proposed by the American Heart Association in 2017 (Table 1).9
Initial coronary artery involvement is evaluated by serial transthoracic echocardiography at the time of diagnosis, and 2 and 6 weeks after the onset of the disease. If abnormal, additional echocardiography is required (maximum of twice weekly) to detect progressive coronary involvement and/or coronary thromboses.9 A comprehensive evaluation of global and segmental ventricular function and an assessment of eventual pericardial effusion or valvular lesions is conducted. Although echocardiography is the method of choice to evaluate cardiac involvement, its accuracy depends on the experience and training of the paediatric cardiologist or sonographer performing the examination.2 Of note, one must remember that an initial normal scan does not rule out coronary lesions, since most CAA usually occur in the second or third week of illness.
Limitations of the method must be kept in mind, such as poor acoustic windows and uncooperative children, for whom sedation, CT angiography or cardiac magnetic resonance (CMR) is of extreme importance. In fact, coronary evaluation is easily and precisely performed by either one of these methods, although it is described that CT, which has a higher resolution and shorter time for image acquisition, is superior to CMR.17 The most common sites for CAA are the proximal left anterior descending artery and proximal RCA, followed by the left main coronary artery, left circumflex, distal RCA and, randomly, in the junction between the RCA and posterior descending coronary artery.2
Treatment During the Acute Phase of KD
The administration of IVIG has altered the natural progression of KD, but cardiovascular injury remains the primary cause of morbidity and mortality.2
The main goal of treating acute illness is to reduce inflammation, heart damage and the risk of thrombosis in people with CAA. Initial treatment is a single high dose of IVIG 2 g/kg within 10 days of the fever onset, followed by a moderate (30–50 mg/kg) to high dose (80–100 mg/kg) of acetylsalicylic acid (ASA) until the patient is afebrile.9 Even though a single dose of IVIG is sufficient to diminish the inflammatory process, approximately 15% of patients will experience IVIG-resistant persistent fever. In these cases, a second dose of immunoglobulin and other immunosuppressive drugs, such as corticosteroids, infliximab or cyclosporine, may be administered. Patients who are resistant to IVIG are at an increased risk of developing CAA.5
A meta-analysis showed that the addition of corticosteroid therapy to IVIG as an initial treatment reduced the rate of CAA and may be a reasonable option for immunoglobulin-resistant patients.18 Unfortunately, these high-risk patients may be difficult to identify, despite the availability of published risk scores.19
Antithrombotic Therapy
Thrombosis is the most feared complication, and prevention efforts are crucial. Low-dose ASA 3–5 mg/kg should be continued in patients without coronary changes for 4–6 weeks after diagnosis. In patients with CAA, therapy will depend on the risk stratification: for the patients with small CAA, monotherapy with low-dose ASA seems to be enough, while for those with moderate aneurysms, combined therapy with a thienopyridine, such as clopidogrel, may be required. Patients with large aneurysms should receive either low molecular-molecular-weight heparin or an oral anticoagulant associated with an antiplatelet agent, keeping the patient in the international normalised ratio range of 2.0–3.0.9 If there is a recent history of giant CAA thrombosis, triple therapy with ASA, a second antiplatelet drug and an anticoagulant agent may be feasible. However, these patients should be closely monitored due to the high risk of bleeding.9
Missed KD Presenting in Adulthood.
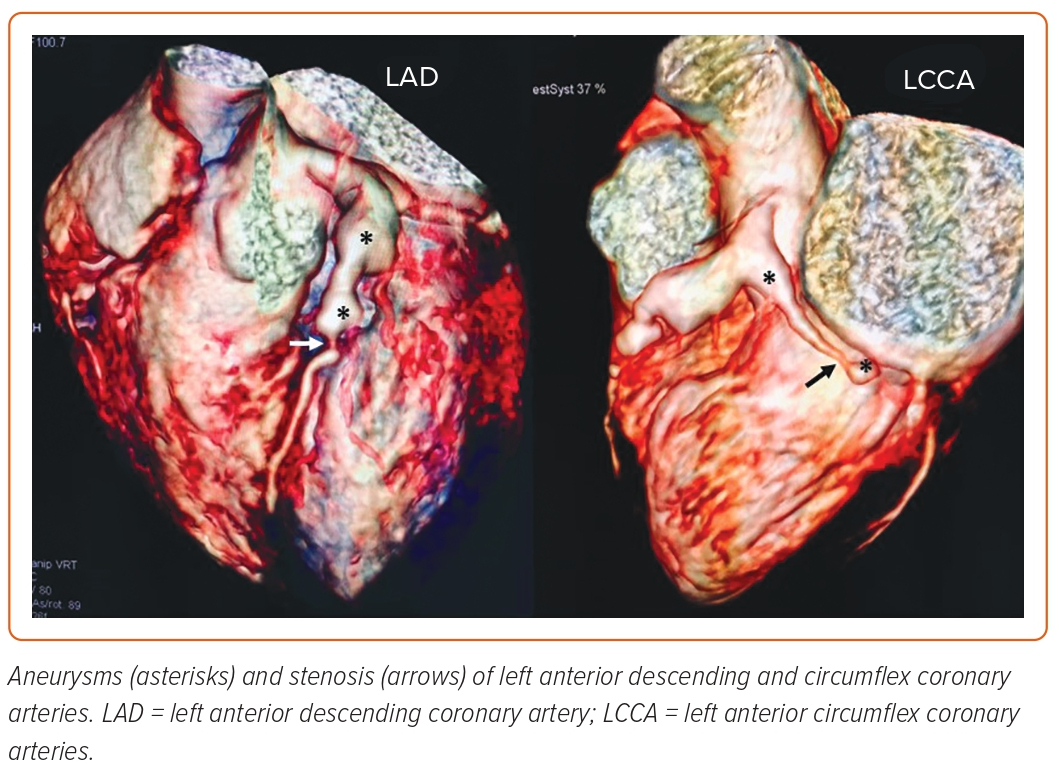
Although paediatricians typically consider the clinical manifestations of KD, these symptoms are not always simple to identify. Aside from persistent fever, the other symptoms may not manifest, and KD may be misdiagnosed as any other viral infection. In fact, this creates a population of young adults with substantial cardiovascular damage who are unaware of their previous KD and risk for acute coronary syndromes or MI (Figure 4).20
If a young person has angina, a MI, ischaemia-induced arrhythmia or a sudden death with proximal aneurysms followed by normal distal segments in an angiography, this could be a sign of undiagnosed KD in the past (Supplementary Material Figures 3 and 4).21
Nowadays, CT angiography and CMR have emerged as non-invasive methods of choice to evaluate KD cardiac involvement. CT angiography is extremely precise to evaluate anatomical aspects of coronary lesions, as well as extremely small aneurysms and thrombi with a very low dose of radiation (<0.3 mSv).22 In contrast, CMR is able to detect both coronary lesions and myocardial function in acute and chronic phases. Stress perfusion could add some important information about the clinical importance of coronary lesions in high-risk patients without ionising radiation.23
Additionally, newer CMR tools, such as the CMR myocardial deformation, demonstrated lower circumferential and longitudinal strain in chronic KD patients, despite the absence of coronary lesions, possibly related to a previous inflammation of the myocardium.24
Currently, invasive angiograms are mostly used whenever percutaneous procedures are required, though there are some negative outcomes of coronary intervention in these patients, which include failure to appreciate the extent of aneurysmal dilatation of a thrombosed artery, resulting in undersizing of a stent, and attempted angioplasty in a heavily calcified artery.1,20 Extensive calcification should be recognised because densely calcified vessels may not be dilatable by angioplasty or successfully stented unless pretreated with rotational atherectomy.20 Multiple artery lesions and associated ventricular dysfunction may require a coronary artery bypass graft or even a heart transplant.9,21
Pregnancy in KD Patients
Pregnancy in KD patients with persistent aneurysms is not associated with an increased risk, according to published data. During pregnancy, women with persistent large coronary arteries should be administered low-molecular-weight heparin and low-dose aspirin. Obstetric considerations should determine the mode of delivery, and CAA alone are not a reason for a caesarian.25,26
Long-term Management
It is not clear if atherosclerotic risk factors affect the long-term progression of KD.27 Despite this, a recent scientific statement from the Japanese Circulation Society recommends eliminating arteriosclerosis-promoting factors in KD patients with coronary artery lesions.2 Lifestyle modifications include quitting smoking, preventing obesity, consuming a healthy diet and exercising.1 Patients with large aneurysms may be required to limit their participation in competitive sports, and patients taking anticoagulants should avoid body-contact sports.1
Due to their beneficial effects on inflammation, endothelial function and oxidative stress, the American Heart Association and Japanese Circulation Society have recently recommended statins as an empirical therapy for KD patients with past or present aneurysms.28 There are data showing that 3 months of short-term statin therapy improved chronic vascular inflammation and endothelial dysfunction in children with KD with minimal adverse effects.29 The Japanese Circulation Society has also recommended renin–angiotensin system antagonists in KD patients with coronary artery lesions, aiming to prevent coronary artery stenosis and atherosclerotic disease.2 The American Heart Association 2017 guidelines suggested β-blockers be considered for KD patients with persistent giant aneurysms, due to their role in the management of the atherosclerotic disease, in addition to their antioxidant effects.9 Finally, it is important to consider that even patients with self-limited coronary dilatation or aneurysms may be at risk for future cardiovascular involvement, and perhaps we should follow these patients, although this may be financially unfeasible.
Conclusion
Although KD patients with CAA are at increased cardiovascular risk and must be monitored for life, we cannot guarantee that patients with self-limiting mild coronary alterations will never experience a KD-related cardiovascular event. Effective prevention of vascular wall derangements and future significant adverse cardiovascular events requires early disease recognition and prompt anti-inflammatory treatment. Even though echocardiography is the method of choice for surrogate KD patients, we must never forget that CT angiography or MRI can provide valuable information, especially in the case of uncooperative children or distal coronary injuries. Finally, the presence of CAA necessitates vigilant management of antithrombotic therapy and continuous monitoring of myocardial ischaemia. In the KD population, accurate identification of coronary artery obstruction due to aneurysms, thrombosis or arterial stenosis can be crucial.
Click here to view Supplementary Material