Major reductions in death rates in acute coronary syndromes (ACS) were achieved with the implementation of percutaneous coronary intervention allowing rapid coronary reperfusion.1 Nonetheless, mortality and morbidity remain substantial during the ensuing five years after an acute coronary syndrome (ACS).2 Indeed, between 1987 and 2006, survival among 30-day survivors of ACS has not improved despite a marked reduction in early mortality and a substantial shift in the epidemiology of MI with the introduction of cardiac troponins in laboratory diagnostic tests.3 Thus, improvements in early diagnosis, risk stratification and clinical management of patients presenting with ACS are needed. ACS comprises the distinct entities of unstable angina (UA), non-ST elevation myocardial infarction (NSTEMI) and ST elevation myocardial infarction (STEMI), as defined by the current European Society of Cardiology (ESC) and American Heart Association/American College of Cardiology (AHA/ACC) guidelines.4–6 Among the tools available for diagnosis, risk stratification and clinical management of ACS, biomarkers figure prominently.7 Troponins as specific markers of myocardial necrosis constitute the main circulating biomarker for the differentiation between UA and NSTEMI,6 and an early invasive strategy in high-risk patients identified by elevated troponin levels is associated with better short- and long-term outcomes.8
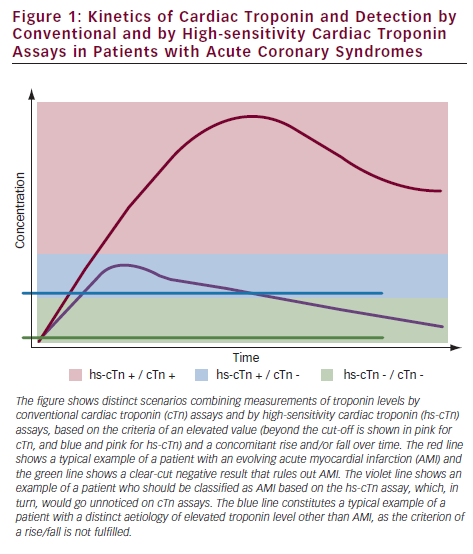
The development of novel high-sensitivity cardiac troponin (hs-cTn) assays for the quantification of cardiac troponins in the circulation at plasma concentrations at the 99th percentile or lower in a reference population with a coefficient of variance of ≤10 % made it possible to fulfil the quality criteria postulated by the Joint ESC/ACCF (American College of Cardiology Foundation)/AHA/WHF (World Heart Federation) Task Force for the Redefinition of Myocardial Infarction.6 In addition to determining the absolute plasma concentrations of cardiac troponins, the rise or fall of cardiac troponin (cTn) levels constitutes an important means to differentiate patients with mild, but relevant, troponin elevations in ACS from those with chronically elevated baseline levels; for example, in stable coronary heart disease.6 Figure 1 shows the distinct scenarios based on elevated absolute concentration and/or a concomitant rise and fall of troponins over time.
Detecting Patients at Elevated Risk and with Subclinical Disease
Cardiac troponin levels detectable by the novel hs-cTn assays are up to 10-fold lower than those detected by the conventional cTn assays.9 Thus, the lower limit of detection of the new high-sensitivity troponin T (hs-TnT) assay by Roche (Elecsys Troponin T®, Roche Diagnostics, Mannheim, Germany) is 0.003 μg/l compared with 0.01 μg/l for the conventional fourth-generation assay corresponding to 0.03 μg/l for the high-sensitivity cardiac troponin T (hs-cTnT) assay.9
Of note, three large population-based cohort studies in apparently healthy individuals using the same hs-cTnT assay showed detectable cardiac troponin T (cTnT) concentrations in a substantial proportion of subjects: 25 % in the Cardiovascular health study,10 66 % in the Dallas heart study11 and 66.5 % in the Atherosclerosis risk in communities (ARIC) study.12 Importantly, elevated hs-TnT levels were also associated with increased mortality in all three studies.
Among those three studies, the ARIC study had the strongest association of hs-cTnT levels with fatal coronary events (odds ratio [OR] 7.59, 95 % confidence interval [CI] 3.78–15.25), hospitalisation for congestive heart failure (OR 5.95, 95 % CI 4.47–7.92) and overall mortality (OR 3.96, 95 % CI 3.21–4.88), but weaker associations with non-fatal coronary events and coronary revascularisations (OR 2.29, 95 % CI 1.81–2.89). This may indicate that elevated high-sensitivity troponin (hs-Tn) levels primarily predict structural heart disease events rather than atherothrombotic events. Indeed, conflicting evidence exists as to whether stress-induced ischaemia is associated with detectable troponin levels using hs-cTn assays.13,14 Beyond silent ischaemia, coronary microvascular dysfunction, structural and functional myocardial alterations as well as apoptosis may account for troponin release from cardiac myocytes. In patients at high risk of cardiovascular events, such as those with congestive heart failure15 or stable coronary artery disease,9 troponin T levels detectable with the high-sensitivity assay but not with the fourth-generation conventional assay were independently associated with adverse cardiovascular outcomes, but not with non-fatal coronary events.
Diagnosis of Patients with Acute Coronary Syndrome
For STEMI, an electrocardiogram (ECG) provides enough information for decision-making, but the distinction between NSTEMI and UA or non-cardiac chest pain requires sensitive and specific biomarkers. Although troponins outweigh other markers of myocardial necrosis in several aspects, conventional cTn tests are disadvantageous in that they can detect a rise in circulating troponin plasma concentration only three to four hours after myocardial damage occurs – similar to creatine kinase myocardial band (CK-MB). Previously, optimal sensitivity of conventional cTn tests could only be achieved six or even nine hours after the onset of myocardial necrosis.6 Therefore, conventional cTn tests provide an insufficient sensitivity to exclude (‘rule out’) AMI (i.e., NSTEMI) in the immediate phase after the onset of chest pain. Accordingly, previous joint guidelines of the AHA and National Academy of Clinical Biochemists recommended the retest of troponin levels in ‘troponin-negative’ NSTEMI patients for at least six hours.16
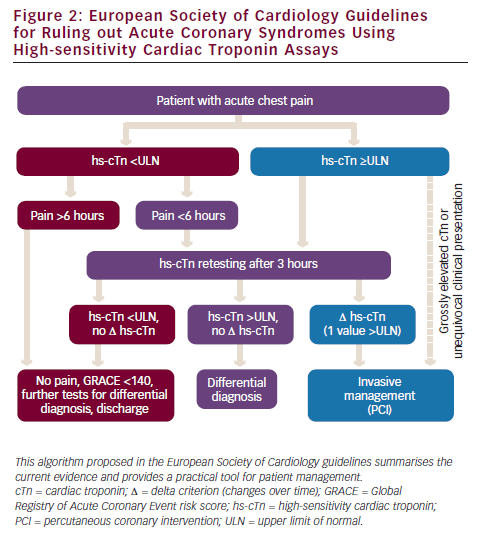
This gap can now be narrowed by the use of novel hs-Tn assays. Owing to the improved sensitivity (∼70 % with cTn assays and ∼90 % with novel hs-cTn assays), a higher negative predictive value is established with a negative hs-cTn test result.17,18 As a consequence, the most recent ESC guidelines for the diagnosis of ACS reduce the time interval for retesting in initially hs-cTn-negative ACS patients to three hours4 (see Figure 2).
However, as a trade-off, the increase in sensitivity is accompanied by a diminished specificity for MI (97 % with cTn assays and 91 % with novel hs-cTn assays) and a reduced positive predictive value (85 % with cTn assays and 77 % with novel hs-cTn assays).17,18 Interestingly, in the ARIC population study, 7.4 % of individuals had cTnT levels ≥0.0014 μg/l – the current threshold level for acute MI (AMI) issued by the manufacturer – and the 99th percentile value was substantially higher at 0.03 μg/l,12 calling into question the versatility of a single cut-off across different ages, gender and renal function. In fact, the diagnostic performance of hs-cTn assays varies substantially in elderly patients, with a suspicion of ACS effecting distinct receiver operating characteristic (ROC)-derived cut-offs.19
The expected decrease in specificity and positive predictive value was recently substantiated by a study in chest pain patients presenting to the accident and emergency (A&E) department that showed a reduced positive predictive value (38 %) for the hs-cTnT test compared with the conventional cTnT test (67–72 %).20
These findings underline the futility of using an absolute threshold level alone and strongly call for additional parameters, such as the kinetics of troponin levels and/or additional biomarkers, for making the diagnosis of MI. Indeed, the former was addressed by a study showing that a doubling of the hs-cTnT concentration within three hours of the first time-point in conjunction with initially elevated hs-TnT levels allowed an increase in the positive predictive value for AMI to 100 %.21 Serial testing using hs-Tn assays at zero and six hours after hospitalisation increases both sensitivity and specificity, and implementation of a delta criterion (% increase) further enhances specificity.22 Absolute changes of cTn levels determined two hours apart have a significantly higher diagnostic accuracy for AMI than relative changes (area under the ROC curve [AUC] for hs-TnT 0.95 versus 0.76; cardiac troponin I ultra 0.95 versus 0.72).23
Three recent studies analysed the combination of copeptin (the arginine–vasopressin prohormone-derived peptide) in conjunction with hs-cTn for the rapid rule-out of MI. The use of pre-specified cut-offs for both markers was associated with an improvement in the rule-out of MI in patients admitted to the A&E department with a likely diagnosis of NSTEMI,24 whereas in patients presenting with acute chest pain, initial negative conventional cTnT levels and a non-diagnostic ECG, the rapid rule-out of MI using hs-TnT could not be further enhanced by adding copeptin (continuous) levels.25 For the identification of patients with MI presenting within three hours of onset of chest pain, a high-sensitivity cardiac troponin I (hs-cTnI) assay delivered an AUC of 0.96 alone, with a small but significant improvement to an AUC of 0.97 (p=0.00397) when copeptin was added.26 In all studies, the specificities remained low.
Risk Stratification of Patients with Acute Coronary Syndrome
The risk stratification of patients with ACS is of paramount clinical importance. Most, but not all, studies that simply compared conventional and sensitive troponin assays found that sensitive troponin assays improve risk prediction of event-free survival at both 30 days and one year.22,27–29 However, the predictive value of sensitive troponin assays must be regarded in conjunction with risk scores that combine the clinical parameters, ECG findings and basic laboratory parameters that have a well-documented clinical value and are recommended by current guidelines.4,5
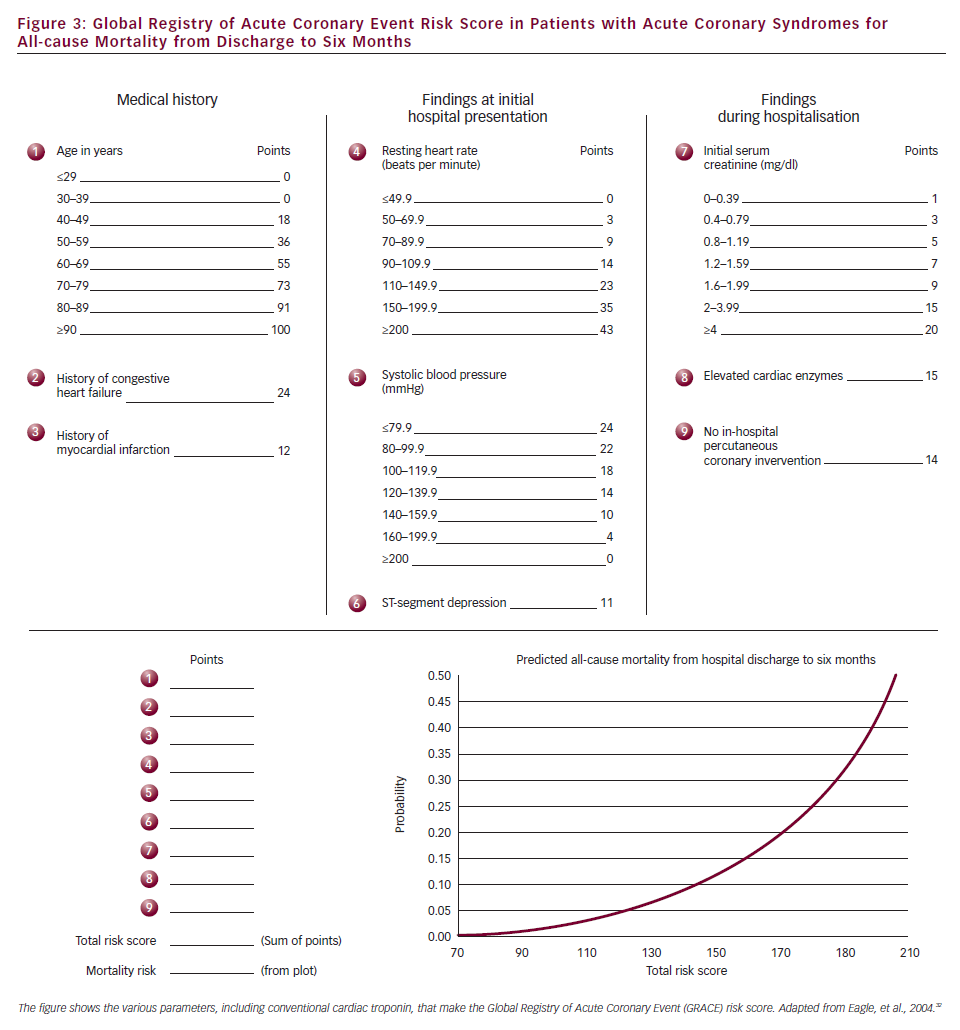
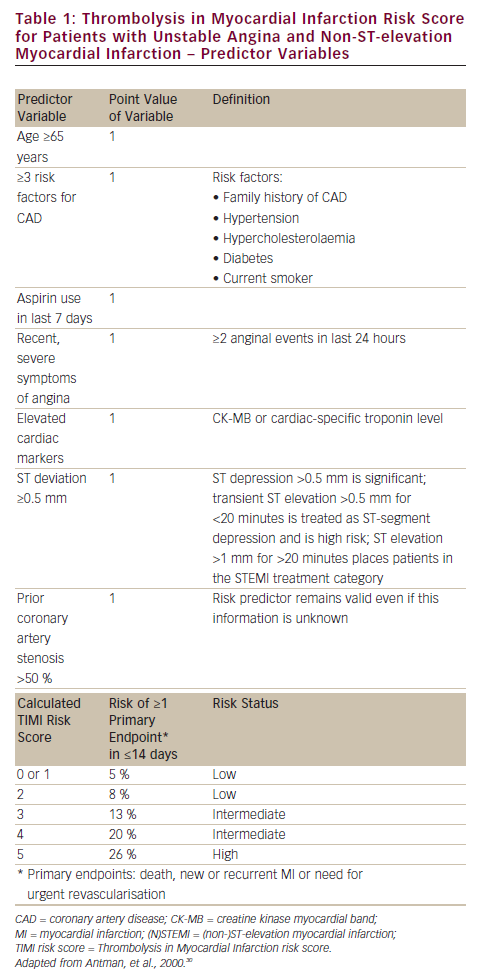
Commonly used is the Thrombolysis in Myocardial Infarction (TIMI) risk score, based on the series of TIMI studies for patients with UA/NSTEMI30 (see Table 1) and STEMI,31 respectively. In turn, the Global Registry of Acute Coronary Event (GRACE) risk score (see Figure 3) is based on registry data and was developed to comprise all ACS entities.32 Clinical risk scores (all of which contain conventional troponin plasma concentration) provide the backbone against which novel biomarkers need to show incremental benefit as assessed by statistical methods using c-statistics, integrated discriminating index and net reclassification improvement (NRI). High-sensitivity troponin assays did not improve risk prediction in studies that included both STEMI and NSTEMI patients.33,34 In patients with acute chest pain,6 high-sensitivity troponin assays improved the prediction of death, but not of subsequent AMI, with an improved reclassification of patients (NRI 0.91) after adjustment for the TIMI risk score.34
A recent multimarker analysis in the MERLIN-TIMI 36 trial of 4,352 patients with NSTEMI, identified a hs-cTn net improvement over the clinical TIMI risk score (excluding troponin) with c-index 0.784 ≥ 0.805 (p=0.005) and NRI 0.389 (p<0.001) for cardiovascular death and similarly for MI, hospitalisation for congestive heart failure and the composite endpoint of cardiovascular death and hospitalisation for congestive heart failure.35 Furthermore, patients presenting with ACS and reclassified into NSTEMI from UA based on hs-Tn against cTn testing correlated with an increased risk of major adverse cardiovascular events and significantly improved risk stratification.27,36 In conclusion, if combined with clinical scores, sensitive troponin assays appear to improve risk prediction in NSTEMI–ACS patients but not, or less so, in STEMI patients.
Clinical Management of Patients with Acute Coronary Syndromes
As of now, only scarce data exist on the effect of introducing hs-Tn assays into clinical practice with respect to clinical management and outcomes after therapeutic intervention. Interestingly, lowering the diagnostic threshold of cardiac troponin I (cTnI) from 0.20 ng/l to 0.05 ng/l by the introduction of a sensitive troponin assay (1,038 patients before and 1,054 after implementation) not only increased the rate of NSTEMI diagnosed in patients with suspected ACS, but also translated into major reductions in morbidity and mortality.37 Future trials will need to examine the benefit of an invasive therapy in AMI patients with elevated troponin, similar to those of the previous era with conventional cTn.38 A prerequisite is the exploration of quantitative cut-offs for hs-Tn and changes over time (delta criterion) to serve as a guidance for therapeutic interventions.
Summary and Conclusions
The availability of hs-cTn tests has improved the diagnostics of patients presenting to the A&E department with chest pain.
Nonetheless, clinical judgement has become evermore important for the correct interpretation of test results that integrate both laboratory parameters (absolute values of hs-Tn and the kinetics thereof) with clinical symptoms and ECG signs of myocardial ischaemia to make the diagnosis of AMI. The increase in sensitivity of hs-cTn tests at the expense of diminished specificity constitutes a challenge to make the diagnosis (‘rule-in’) of AMI. This important topic is currently addressed in two ways:
- by analysing the absolute/relative increase in hs-Tn concentration over the time necessary to make the diagnosis of AMI; and
- by combining the hs-Tn test with an ideally disease-specific complementary biomarker to enable the diagnosis of AMI at a single time-point.
Evidence is accumulating that hs-cTn assays improve short- and long-term prediction, at least in NSTEMI patients, beyond clinical risk prediction rules, which include cTn levels measured by conventional assays. In addition, beyond risk estimation for elevated hs-cTn concentration, the role of hs-cTn testing in the choice of therapeutic intervention and its effects on clinical outcomes are being analysed. Finally, the underlying mechanism for the stronger association of elevated troponin levels with structural heart disease events rather than with atherothrombotic events is of major interest.







