Hypertension is one of the most common chronic diseases in the world. Despite the availability of many treatment options, a large proportion of the hypertensive population still fails to achieve long-term blood pressure (BP) control.1 According to the most recent guidelines of the European Society of Hypertension (ESH), true resistant hypertension (tRH) is defined as systolic BP (SBP) or diastolic BP (DBP) that remains ≥140 mmHg or ≥90 mmHg, respectively, despite appropriate use of lifestyle measures and use of optimal or maximally tolerated doses of a three-drug combination comprising a renin–angiotensin–aldosterone (RAAS) blocker (either an angiotensin-converting enzyme inhibitor or an angiotensin receptor blocker), a calcium channel blocker and a thiazide/thiazide-like diuretic.2 This definition of RH also includes those patients taking four or more drugs to maintain BP <140/90 mm Hg, as well as the category of refractory hypertension, referred to as uncontrolled BP despite five or more drugs of different classes, including a diuretic.3
The American Heart Association (AHA) and American College of Cardiology (ACC) identify resistant hypertension (RH) at a lower BP threshold (i.e. 130/80 mmHg). The AHA/ACC also propose the term ‘controlled RH’ to indicate the presence of BP effectively controlled by four or more antihypertensive agents.4,5 According to the latest ESH guidelines, various causes of pseudo-resistant and secondary hypertension must be investigated and ruled out to define tRH. Moreover, inadequate BP control should be confirmed by out-of-office BP measurement.2 If there are insufficient data on medication dose, adherence to treatment, or out-of-office BP levels, pseudo-resistant hypertension cannot be ruled out and RH is defined apparent RH (aRH).5
RH is associated with increased cardiovascular (CV) morbidity and mortality. Patients with RH generally require more frequent medical examinations, diagnostic tests and medication, resulting in increased healthcare costs and economic burden.6,7 Appropriate management of patients with suspected RH is crucial to avoid misdiagnosis and ensure adequate treatment.
The aim of this review is to provide an overview of RH, including epidemiology, pathophysiology, diagnostic procedure and the latest developments in therapeutic strategies.
Epidemiology
The global burden of RH is relevant, with approximately 100–500 million people globally estimated to be affected.5 Establishing the exact prevalence of RH is challenging. Several factors influence RH prevalence, such as the clinical setting, antihypertensive drugs used, adherence to treatment, method of BP measurement and the definition of target BP.5,8,9 In a meta-analysis including data from 91 cohort or cross-sectional studies comprising more than 3.2 million patients, the prevalence of RH was about 10% among patients treated for hypertension.10 In a more recent Italian cohort of patients referred to primary care physicians, >20% of patients with hypertension actually had RH, defined as treatment with three different antihypertensive agents with recorded office BP ≥140/90 mmHg, or patients taking ≥4 medications.11
In an analysis of more than 2.4 million individuals in the US, a prevalence of apparent RH of 8.5% was observed, lower than previously reported (12–15%), but with a high burden of comorbidities. Identification of differences in pharmacotherapy between patients with controlled and uncontrolled RH, particularly lower rates of mineralocorticoid receptor antagonist (MRA) use, may help define potential opportunities to improve care and lower CV risk.12
After strictly applying the above definition of RH, a reasonable estimate of disease prevalence could be around 5% of the overall population with hypertension.2 RH prevalence is higher among patients with diabetes and hypertension-mediated organ damage (HMOD), including albuminuria, left ventricular hypertrophy and chronic kidney disease (CKD). 10,13–18 Other clinical conditions significantly associated with RH are the number of antihypertensive drugs used, male sex, older age, obesity, black African origin, low income, depression and a 10-year CV Framingham risk score >20%.13–15 Obstructive sleep apnoea (OSA) is also very common among patients with RH.19
Using data from NHANES, the prevalence of refractory hypertension was reported to be in the range of 0.3% to 0.9% across multiple cycles. Among patients prescribed five or more antihypertensive drugs, the prevalence of refractory hypertension was 34.5%.20 The demographic and clinical factors associated with refractory hypertension were advancing age, lower household income, black ethnicity, CKD, albuminuria, diabetes, prior stroke and coronary heart disease.
Pathophysiological Mechanisms
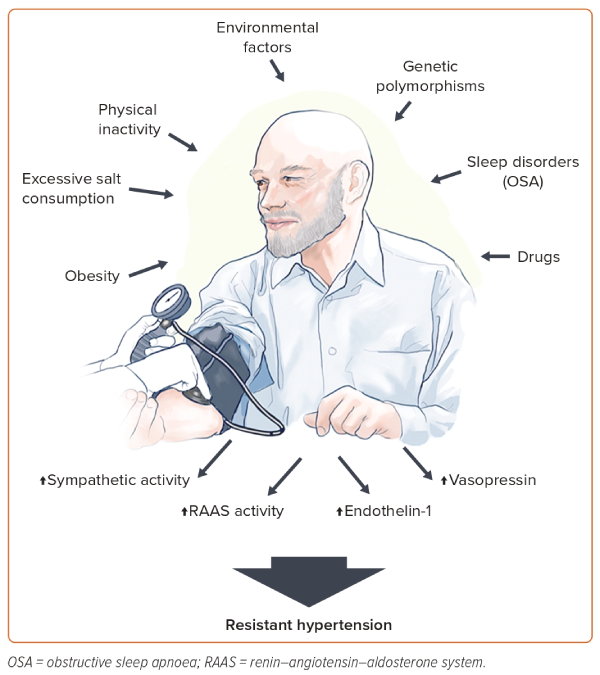
The pathophysiology of RH involves an interplay between several neurohumoral factors, including increased sympathetic activity and levels of aldosterone, endothelin-1 and vasopressin.21–24 These factors induce volume and sodium overload and contribute to increased peripheral vascular resistance, arterial stiffness and HMOD occurrence.21,25
Several conditions contribute to such mechanisms (Figure 1). Obesity is an important risk factor for RH development.14 Findings from NHANES demonstrated that BMI ≥30 kg/m2 approximately doubles the risk of aRH.26 In particular, visceral adiposity plays a fundamental role in RH occurrence through a variety of mechanisms, including enhanced salt sensitivity, vascular dysfunction and activation of the sympathetic nervous system and RAAS.27,28 Both reduced physical activity and lower physical fitness are independent risk factors for hypertension, although there is a paucity of data on patients with RH.
In the REGARDS cohort, self-reported inactivity was not predictive of RH.29 On the other hand, indirect evidence from randomised controlled trials (RCTs) on lifestyle interventions suggests an important role of physical inactivity in the development of the disease.30,31 Environmental factors may also contribute to the increased risk of RH. Using the NHANES data, Chen et al. recently identified that blood levels of heavy metals, such as lead and cadmium, were associated with increased prevalence of RH.32
Several studies have also reported new or exacerbated RH in patients with previous severe acute respiratory syndrome coronavirus 2 infection. The exact mechanism linking such conditions may involve the binding of the spike protein to angiotensin converting enzyme-2 (ACE2) receptors on the cell surface leading to ACE2 downregulation and failure of the counter-regulatory RAAS axis.33 However, these aspects have yet to be clearly demonstrated.34
Sleep is a crucial component of overall health and disruptions in sleep patterns has been associated with an increased risk of hypertension and CV disease as a result of sympathetic overactivity.35,36 OSA is a common sleep disorder characterised by repetitive episodes of nocturnal breathing cessation due to upper airway collapse. It is a particularly strong risk factor for the development of RH.19 Recurrent hypoxia occurring in patients with OSA triggers endothelial dysfunction and activates the RAAS and sympathetic systems, leading to systemic inflammation and oxidative stress that contribute to RH and HMOD occurrence.37,38
Several classes of drugs can increase BP through various mechanisms, such as increased systemic and renal vasoconstriction, sodium retention and angiotensin biosynthesis. These pharmacological agents include nonsteroidal anti-inflammatory agents, oral contraceptives, immunosuppressive treatments such as cyclosporine and tacrolimus, antineoplastic drugs targeting the vascular endothelial growth factor pathway, steroids and antidepressants.39–44
There is limited evidence on RH heritability. Most genetic research on RH has been limited to candidate genes and lack adequate sample sizes.45–49 One of the genes potentially involved in RH susceptibility is the angiotensinogen (AGT) gene, encoding a protein that is a precursor to angiotensin II.47 The M235T polymorphism of the AGT gene, in particular, is associated with increased plasma angiotensinogen levels leading to increased risk of hypertension.50,51 Genetic variants in the adrenergic receptor genes may be also linked to the development of RH.52 Larger studies in well-characterised individuals with RH are needed to clarify these aspects.
Clinical Implications
Patients with RH have an increased risk of developing HMOD, CKD and premature CV events.17,18,53–55 In a retrospective study including more than 200,000 patients with hypertension, RH was associated with higher rates of MI, heart failure, stroke, or death over a median of 3.8 years. Differences in CV events were mainly driven by an increased risk of CKD development in patients with RH compared with those with hypertension responsive to treatment.54 Similar findings were reported by another observational study including over 400,000 subjects.6 Prospective studies using ambulatory BP monitoring (ABPM) also suggested an increased risk of CV events in patients with RH.56,57
In the REGARDS study, uncontrolled aRH was associated with increased risk of coronary heart disease (HR 2.33; 95% CI [1.21–4.48], but not stroke (HR 1.05; 95% CI [0.61–1.81]) or all-cause mortality (HR 1.15; 95% CI [0.91–1.45]).58 Interestingly, another study of more than 40,000 patients with RH found that BP control significantly reduced rates of stroke and coronary artery disease, albeit to a lesser extent than in subjects without RH.59 Compared to patients with hypertension controlled by treatment, patients with uncontrolled RH may, therefore, have worse outcomes regardless of BP control and require closer medical monitoring.
In this regard, patients with refractory hypertension may be at particularly high risk for long-term adverse events. In the CRIC study, despite no significant differences in all-cause mortality, patients with refractory hypertension had a significantly higher risk of CV and renal outcomes than subjects with RH.60
Diagnostic Work-up: Ruling out Apparent Resistant and Secondary Hypertension
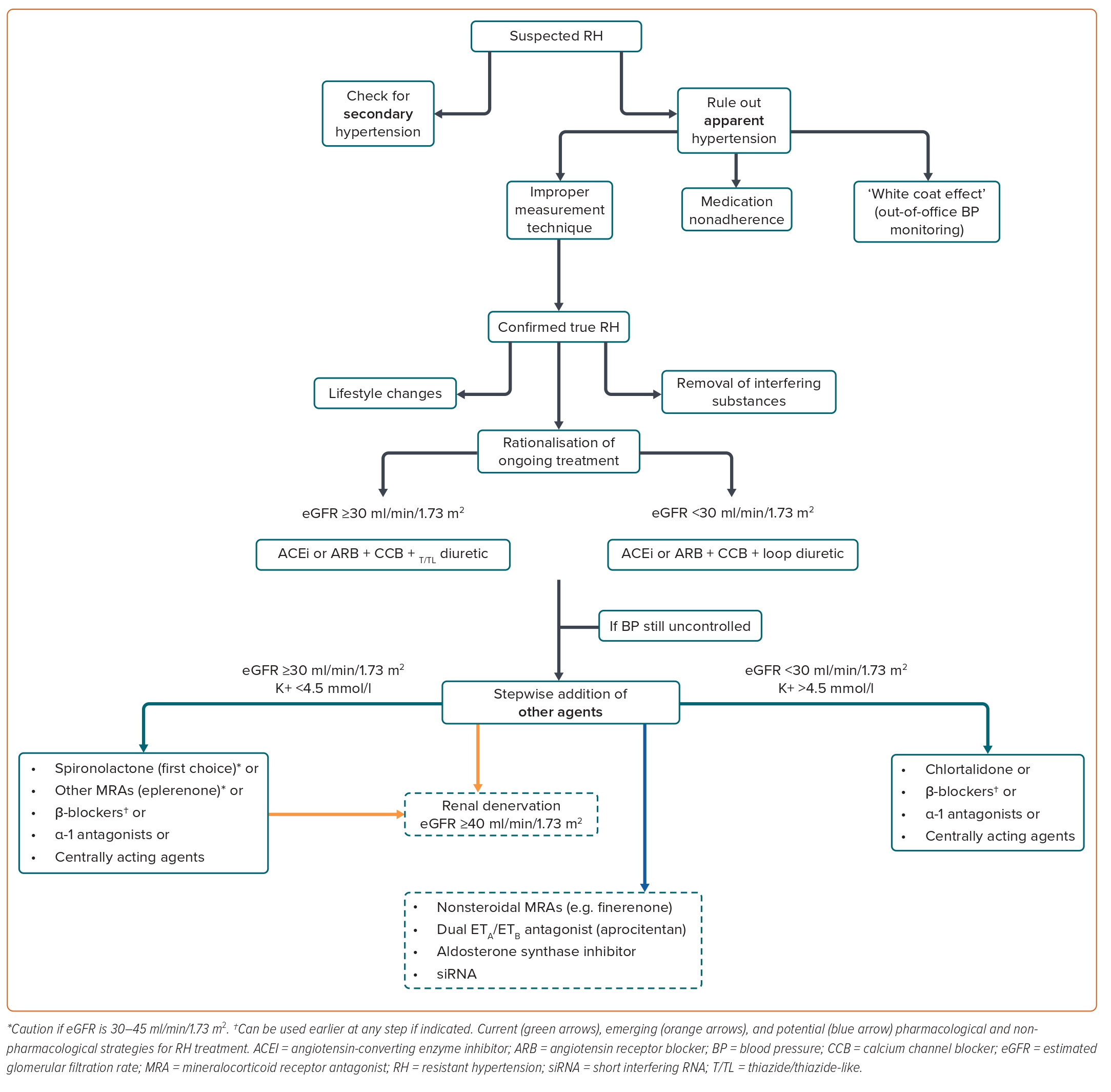
The diagnostic work-up for patients with suspected RH is shown in Figure 2.
Secondary causes of hypertension must be checked, according to the patient’s medical history and physical examination. Appropriate laboratory or imaging testing may be also warranted, according to the most recent ESH recommendations.2 Secondary causes account for 5–10% of hypertension cases, particularly primary aldosteronism and atherosclerotic renal artery stenosis.22,61
Primary aldosteronism is the most common endocrine cause of secondary hypertension in patients with suspected RH. In a sub-study of PATHWAY-2 trial, a randomised, double-blind crossover trial on patients with RH treated with different classes of antihypertensive agents, including spironolactone, 25% of the patients were deemed to have inappropriately high aldosterone concentrations, suggesting that aldosteronism probably goes underdiagnosed in many cases of RH.62 Atherosclerotic renal artery stenosis is the most common cause of renovascular hypertension. In case of clinical suspicion, renal artery Doppler ultrasound should be performed as first-line imaging, followed by MRI and/or CT angiography to confirm the diagnosis.63
Another crucial step is to rule out aRH by excluding improper measurement techniques, medication nonadherence and the ‘white-coat effect’. The patient’s history must be carefully recorded, including lifestyle features, alcohol and dietary sodium intake, concomitant drugs and sleep history. The presence of OSA should be checked, because this condition is frequently involved in the development of hypertension. To estimate the OSA risk, several tools are currently available, including the Berlin Questionnaire, Epworth Sleepiness Scale and the STOP-Bang Questionnaire.64 The disease should be then confirmed with appropriate tests like in-lab attended sleep study or polysomnography. The nature and dosing of the antihypertensive agents used should be assessed. To confirm RH, drugs should be prescribed above their starting dose and not necessarily at the maximum recommended dose. Once the diagnosis of tRH is confirmed, the patient should be carefully evaluated to identify associated risk factors and HMOD presence.2
Errors in BP measurement can account for RH misdiagnosis. Therefore, proper patient preparation, adequate environmental conditions, use of an appropriately sized cuff and the technique of BP measurement are crucial aspects to consider. Accurate BP measurement, both at home and in the office, requires an intentional approach to measurement and some level of training. The use of a standardised office BP methodology is of utmost importance to avoid errors. A recent ESH statement provides recommendations for proper measurement, patient position, device, measurement schedule and interpretation of office BP results.65 Spurious BP increase associated with brachial artery calcification should be ruled out in this setting, particularly in the elderly and in patients with advanced CKD.2
Failure to identify medication nonadherence contributes to overestimating the prevalence of tRH and may have important consequences in clinical research. A recent post hoc analysis by Kario et al. demonstrated that almost half of the participants with RH included in the REQUIRE study exhibited poor adherence to medical treatment.66 Among these subjects, those receiving catheter-based ultrasound renal denervation displayed unchanged adherence over time, whereas subjects undergoing sham procedure showed a trend towards increased adherence and a significant BP reduction during the study course, possibly explaining the negative findings of the study.66
In a meta-analysis of 24 studies including patients with aRH, the mean prevalence of nonadherence was 31%, ranging from 3% to 86%.67 Similar results were provided by another meta-analysis by Bourque et al.68 Several direct and indirect tools are currently available, although there is no gold standard.69 In general, direct methods like witnessed drug intake, the Medication Event Monitoring System and drug screening of urine or blood are much more effective, whereas indirect methods such as questionnaires or pill counts proved to be unreliable.70
Pharmacodynamic markers of exposure to drugs, such as bradycardia occurrence on β-blockers, increased urine N-acetyl-seryl-aspartyl-lysyl-proline concentration on angiotensin-converting enzyme inhibitor, increased circulating levels of uric acid on diuretics and renin concentration on diuretics or RAAS blockers, as well as drug-related side-effects, may have limited specificity in this setting.71 As adherence fluctuates over time and tends to decrease progressively, repeated adherence measurements should be considered.72
Out-of-office BP monitoring is generally required to exclude the white-coat effect and confirm the diagnosis of RH. The white-coat effect is defined as office BP above the target in a patient on at least three antihypertensive drugs but with appropriate levels of out-of-office BP. The latter is measured with 24-hour ABPM. APBM is not only a fundamental tool for diagnosing RH, but it may also play a role in predicting future CV events in subjects with RH.73 When ABPM is not readily available, home BP monitoring can be considered.2 Self-measured home SBP has been found to correlate with average daytime SBP assessed by ABPM.8
Treatment Options for Resistant Hypertension
Once the diagnosis is established, RH management remains challenging. Effective treatments should combine lifestyle changes and removal of interfering substances, optimisation of ongoing treatment and a sequential introduction of antihypertensive drugs on top of triple therapy.
Further options, such as renal artery denervation, may be considered in selected cases.2 Given the association with various comorbidities and the need for multiple and complex drug regimes, patients with RH should be referred to a hypertension specialist or even to a specialised hypertension centre if necessary. A dedicated follow-up program is mandatory.2
Lifestyle Changes
Growing evidence supports the role of lifestyle changes in RH management. In a recent network meta-analysis, lifestyle interventions were the most effective non-pharmacological treatment in the setting of RH, lowering office SBP by −7.26 mmHg (95% CI [−13.73, −0.8]).
Dietary Approaches to Stop Hypertension (DASH) is a dietary intervention that emphasises the consumption of whole grains, vegetables, fruits and low-fat dairy products, while limiting the intake of saturated fats, processed foods and added sugars. In several trials, this strategy has been shown to be effective in reducing BP in patients with RH.74 Similar benefits have been observed with a Mediterranean diet.75
High sodium intake increases the risk of treatment-RH.76 However, direct evidence of the benefits of sodium restriction in the RH population is scarce. In a study including 15 patients with RH, self-performed dietary sodium restriction led to a significant reduction in BP and urinary sodium excretion after 2 weeks.77 In a previous RCT, a low-salt diet reduced office SBP and DBP by 22.7 and 9.1 mmHg, respectively, though the small sample size, the unblinded administration of salt diets and the short duration of dieting periods strongly limit the interpretability of such results.78
Regular physical activity improves CV health by reducing inflammation and improving lipid profile.79 In the setting of RH, both aerobic and resistance physical activity have been found to reduce SBP and DBP.30,79 In a study of 53 patients with RH, a 12-week program of moderate aerobic exercise reduced ambulatory SBP by 7.1 mmHg compared with usual care.31 In the TRIUMPH study, a comprehensive lifestyle intervention in which participants received instruction from a nutritionist and exercise in cardiac rehabilitation facilities reduced both office BP and ABPM values while improving biomarkers of CV disease and psychological functioning, compared with standard care.80,81 In the EnRicH trial, a 12-week moderate-intensity aerobic exercise training program led to ambulatory SBP and DBP reduction by 7.1 and 5.7 mmHg, respectively.31 Compared with usual care, this strategy also lowered central BP while improving angiotensin II and superoxide dismutase circulating levels.82
Evidence on the effects of weight loss in patients with RH remains limited. In patients with obesity, glucagon-like peptide-1 agonists modestly lower BP and improve CV risk profile in patients with diabetes or established CV disease.83–85 In a sub-analysis of the GATEWAY trial, including patients with hypertension and a BMI between 30 and 39.9 kg/m2, RH prevalence significantly decreased following randomisation to bariatric surgery.86 Interestingly, postoperative BP reduction preceded weight loss in treated patients, suggesting that further changes are responsible for these benefits including improvements in sympathetic overactivity, sodium and water homeostasis and inflammation.87
No data are currently available on the effects of avoiding smoking in RH.88
In patients with RH and OSA, continuous positive airway pressure (CPAP) therapy has been demonstrated to decrease BP.37 In a Spanish trial, subjects who benefited most from CPAP treatment were those with uncontrolled baseline BP and more severe OSA-related nocturnal hypoxia.89 A subsequent sub-analysis of the same study found that reduction in 24-hour BP levels was significant only in patients with a rising profile and no-dipping pattern.90
Pharmacological Strategies
According to the latest ESH guidelines, effective pharmacological treatment of RH should combine rationalisation of current medications and the sequential addition of antihypertensive agent to the existing triple therapy.2 The suggested therapeutic approach for patients with confirmed RH is shown in Figure 2.
To improve ongoing treatment strategies, combination therapies should be used at optimal or maximally tolerated doses while considering patient’s age, indications for specific drug classes, existing comorbidities and risks of drug–drug interactions. Single pill combinations should be preferred to reduce pill burden and improve medication adherence. Increasing the intensity of diuretic therapy may be necessary, particularly in the elderly, in subjects of black African origin or with CKD. In patients with an estimated glomerular filtration rate (eGFR) ≥45 ml/min/1.73 m2 receiving hydrochlorothiazide, the drug dosage can be increased. As hydrochlorothiazide loses its ability to induce predictable natriuresis when eGFR is <45 ml/min/1.73 m2, the drug can be switched to a longer-acting thiazide-like diuretic (e.g. indapamide or chlorthalidone) in such cases. If eGFR is <30 ml/min/1.73 m2, indapamide should not be used, whereas chlorthalidone doses may need to be increased to 50 mg daily. If this strategy is not sufficient, thiazide diuretics may be replaced by loop diuretics (e.g. furosemide, bumetanide, or torsemide).2 Of note, in a recent RCT on patients with stage 4 CKD (with a mean eGFR of 23.2 ± 4.2 ml/min/1.73 m2), a mean reduction in ambulatory SBP of 10.5 mmHg was reported in patients randomised to chlorthalidone compared with placebo, supporting the efficacy of this drug in severe CKD.91
After optimising the ongoing therapy, a stepwise addition of other agents should be considered if BP is still not at goal. Based on the results of the PATHWAY-2 trial and other studies, patients with RH should receive the MRA spironolactone 25–50 mg/day as fourth-line treatment.92–94 In the ReHOT trial, which randomised spironolactone versus clonidine as the fourth drug in patients with RH, patients on spironolactone displayed a larger decrease in 24 hours SBP and DBP.95 In a recent network meta-analysis including 24 studies and about 3,000 individuals, among all comparators, spironolactone was the most effective treatment to reduce office SBP (−13.30 mmHg) and 24 hours SBP (−8.46 mmHg) in patients with RH.96
Real-world evidence from the US comprising more than 80,000 patients with RH found a non-significant reduction in the rates of stroke and MI in patients taking spironolactone compared to β-blockers as fourth-line treatment, though the risk of hyperkalaemia and kidney function worsening was significantly higher with the former.97 The risk of hyperkalaemia with spironolactone is a cause for concern, particularly in patients with CKD taking RAAS blockers. According to current guidelines, spironolactone should be used with caution in patients with an eGFR <45 ml/min/1.73 m2 when plasma potassium concentration is >4.5 mmol/l, as these were exclusion criteria in the PATHWAY-2 trial. In patients with heart failure, particularly those with reduced ejection fraction, the drug is recommended in light of the well-established benefits of spironolactone in this clinical setting, although contraindicated with an eGFR <30 ml/min/1.73 m2.2,98
After treatment initiation, plasma potassium and eGFR must be closely monitored, at least annually or at 3 to 6-month intervals thereafter.2 Given its affinity for other receptors, such as the progesterone and androgen receptors, spironolactone may also be associated with antiandrogenic side-effects, resulting in gynecomastia, breast tenderness and sexual impotence in men and menstrual abnormalities in women.99 The overall suboptimal tolerability profile of spironolactone may explain its low prescription rate and the low persistence on treatment in real-life settings.
Eplerenone is a more selective MRA that can be used as an alternative to lower BP. However, it is less potent than spironolactone, whereas its cost remains a significant barrier to its widespread use in clinical practice.100
More selective nonsteroidal MRAs, such as finerenone (approved for the treatment of patients with diabetic CKD), esaxerenone (approved for the treatment of hypertension in Japan) and ocedurenone (undergoing clinical trials for the treatment of RH in patients with CKD) might provide further alternatives to spironolactone in the future.100,101
When spironolactone and other MRAs are not tolerated or contraindicated, alternatives include amiloride, which was found to be as effective as spironolactone in reducing BP when used at high dosages (10–20 mg/day), β-blockers like bisoprolol 5–10 mg/day, α-1 antagonists such as doxazosin extended release 4–8 mg/day, and centrally acting agents such as the α-adrenergic receptor agonist clonidine 0.1–0.3 mg twice daily.62
In the ReHOT trial, clonidine showed a similar reduction in office and ambulatory BP compared to spironolactone.95 Conversely, in the PATHWAY-2 trial, bisoprolol and doxazosin reduced BP less effectively than spironolactone, although the difference was small.92 α-1 antagonists are associated with an increased risk of orthostatic hypotension, syncope, falls and fractures.102 Furthermore, although controversial, in the ALLHAT trial, doxazosin use was associated with a higher incidence of heart failure compared to chlorthalidone, leading to premature discontinuation of this treatment arm.103 Clonidine use is also associated with several side-effects, such as dry mouth, sedation and dizziness.104 Moreover, the risk of rebound hypertension after drug discontinuation or during periods of nonadherence is a cause for concern. Using a sustained release transdermal formulation may attenuate some of these issues.
Of note, the abrupt cessation/withdrawal of β-blockers, which are used more extensively than clonidine in the hypertensive population, is associated with an even more critical risk of rebound hypertension. This aspect must be carefully considered when prescribing these drugs.
Direct vasodilators, such as hydralazine or minoxidil, should be used with caution, as they may cause severe fluid retention and induce tachycardia through reflex sympathetic activation.2
In patients eligible for treatment with sodium-glucose cotransporter 2 inhibitors (SGLT2I), using these agents on top of antihypertensive therapy may have an additional moderate BP-lowering effect.105 In a post hoc analysis of the EMPA-REG OUTCOME trial, SBP was more frequently controlled with empagliflozin compared to placebo at week 12 in patients with presumed RH (38% versus 26%, respectively), with a mean difference in SBP change of −4.5 (95% CI [−5.9, −3.1]) mmHg between groups. Furthermore, this drug significantly reduced the risk of heart failure leading to hospitalisation, worsening renal function and CV death, both in patients with and without presumed RH.106 Similar data were found with canagliflozin.107 However, it should be noted that the above analyses were not prespecified to assess such outcomes and there is insufficient information to determine whether and how many patients with presumed RH had true RH in the studies.
Lastly, in a post hoc analysis of the PARAGON HF trial, sacubitril/valsartan combination reduced BP in patients with RH, despite treatment with at least four antihypertensive drugs including an MRA.63 Such findings were also reported in a phase II trial of patients with hypertension.108 However, no data are currently available from clinical studies conducted specifically on patients with RH.
Future Pharmacological Approaches
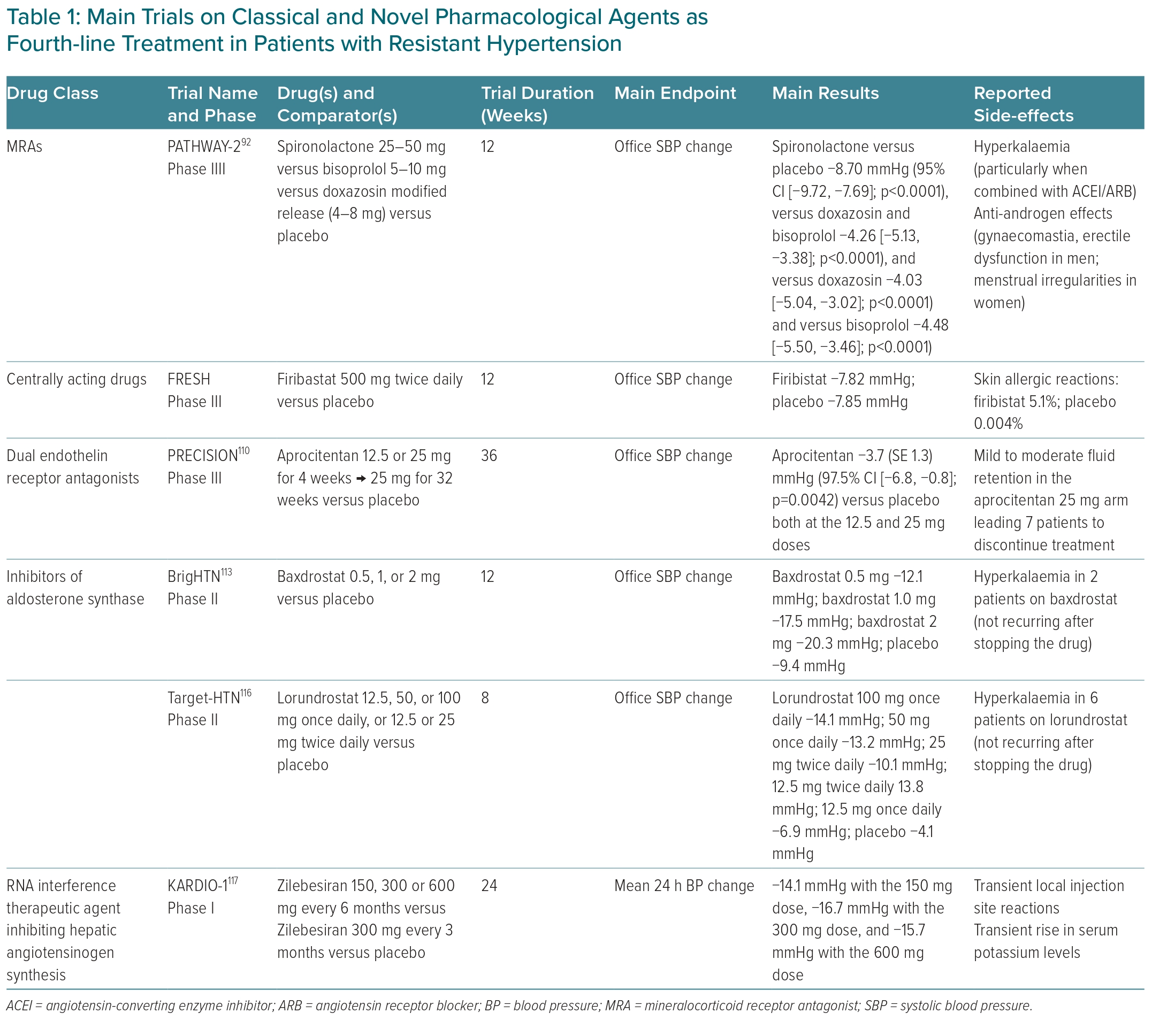
Several novel antihypertensive agents are being explored for the treatment of RH (Table 1). Endothelin has been implicated in the pathophysiology of hypertension for several years and endothelin receptor antagonists have become an integral part of the treatment of pulmonary arterial hypertension.109 Aprocitentan is a dual endothelin antagonist directed against endothelin-1 receptors ETA and ETB that may have a future role in RH management, particularly in patients at high risk of hyperkalaemia and renal failure with MRAs.
The PRECISION trial was a multicentre, blinded, randomised, parallel-group, phase III study including patients with uncontrolled RH on triple antihypertensive therapy including a diuretic randomised to aprocitentan versus placebo.110 Compared with the latter, both 12.5 and 25 mg aprocitentan dosages significantly reduced office SBP at 4 weeks (−3.7 and −3.8 mmHg, respectively), with a sustained effect at week 40. Although well tolerated overall, mild-to-moderate oedema or fluid retention occurred in 9% and 18% of the patients receiving aprocitentan 12.5 and 25 mg, respectively, leading to drug withdrawal in some cases.23,110
Baxdrostat (CIN-107) is a highly selective, competitive and potent inhibitor of aldosterone synthase with no effects on the highly homologous enzyme 11β-hydroxylase (CYP11B1), which is responsible for cortisol synthesis.111,112 The advantage of this drug resides in the possibility of reducing aldosterone synthesis instead of blocking its effects on mineralocorticoid receptors. The phase II BrigHTN showed dose-dependent changes in office SBP of −20.3, −17.5 and −12.1 mmHg in patients randomised to baxdrostat at the dose of 2, 1 and 0.5 mg, respectively. The difference in the change in office SBP observed between the 2-mg group and the placebo group was −11.0 mmHg. No serious adverse events were reported, whereas severe hyperkalaemia was attributed to baxdrostat in two patients.113 Notably, another phase II study, the HALO trial failed to demonstrate the efficacy of baxdrostat in lowering BP in patients with uncontrolled hypertension.114 Possible explanations for this discrepancy in terms of outcome include differences in studies duration and baseline clinical characteristics of the patients enrolled, such as BP levels, rates of uncontrolled diabetes and eGFR values.115 The benefits and safety of baxdrostat thus need to be confirmed in phase III trials over longer periods. The drug is currently undergoing clinical trials in patients with hypertension, hypertension with CKD (NCT05432167) and primary aldosteronism (NCT04605549).
The Target-HTN study also demonstrated the efficacy of lorundrostat in reducing both BP levels and serum aldosterone independently of baseline plasma renin activity, at the higher doses tested, with a remarkable interindividual variability in the in the BP-lowering effect. Like baxdrostat, inhibition of the aldosterone synthase with lorundrostat did not modify cortisol levels.116
The use of short interfering RNA (siRNA) to suppress the synthesis of hepatic AGT represents a novel opportunity in the treatment of RH through improved durability and innovative targeting.117 In the phase I/first-in-humans studies, the first-in-class AGT-targeting siRNA, zilebesiran, was well tolerated and dose-dependently reduced serum AGT levels (by >90%), with sustained effects on BP levels (reduction in 24-hour SBP >10 mmHg by week 8, and lasting 6 months at ≥200 mg dosage), without worsening of renal function.117
Renal Denervation
Experimental devices and other interventions are being explored in patients with RH to reduce BP, including baroreflex activation therapy, renal denervation, endovascular baroreflex amplification therapy, carotid body ablation and cardiac neuromodulation therapy. Baroreflex activation therapy, mimicking the natural baroreflex mechanism, stimulates the baroreceptors to send signals to the brain, leading to a reduction in sympathetic nervous system activity and subsequent lowering of BP.118,119 Renal denervation involves disrupting renal sympathetic nerves to reduce their stimulatory effect on BP. By using catheter-based techniques, nerves in the renal arteries are ablated, leading to decreased sympathetic activity, lower BP, decreased renin activity and increased renal blood flow.
It is important to underline that while these interventions are promising, their effectiveness and safety may vary. Most evidence concerns renal denervation in RH, which is a primary indication for this intervention. The SYMPLICITY HTN-2 trial randomly allocated patients to undergo immediate renal denervation or continue with usual treatment with the possibility of receiving delayed procedure. The study showed that renal denervation is safe, with lasting reduction in BP to 1 year in patients with severe hypertension and SBP >160 mmHg.120 However, SYMPLICITY HTN-3, the first randomised, sham-controlled trial, failed to demonstrate a significant effect on BP in patients with severe RH.121 This might be due to several factors, such as a longer history of hypertension, more severe and non-reversible HMOD and arterial stiffness, high failure rates, operator experience and medication nonadherence upon completion of the procedure.
Recent studies, particularly the SPYRAL HTN-OFF MED trial, have highlighted the effectiveness of RDN in reducing both office and 24-hour BP among patients with lower BP values without the use of medications.122 Additionally, findings from the SPYRAL HTN-ON MED trial underscored significantly lower BP values at the 6-month mark within the renal denervation group, further emphasising its potential benefits with the improved multi-electrode radiofrequency Spyral catheter (Medtronic).123 A parallel investigation conducted by Azizi et al. affirmed the positive impact of renal denervation in terms of ABPM by employing an innovative ultrasound catheter design and implementing a more stringent medication protocol.124 In the RADIANCE-HTN Trio study, the authors showcased a noteworthy decrease of daytime ambulatory SBP of 5.8 mmHg in comparison to the control group, indicating a modest yet significant therapeutic advantage.124
Following the release of the 2018 European Society of Cardiology and ESH guidelines for the management of hypertension, multiple well-executed sham-controlled trials have been published, underscoring the safety and efficacy of renal denervation in lowering BP.125 Therefore, renal denervation emerges as an additional viable treatment alternative for adult patients grappling with uncontrolled RH, as indicated by the latest European guidelines stating the potential utility of this strategy in the context of RH with a class II recommendation, provided that the eGFR is >40 ml/min/1.73 m2.2
In November 2023, the Food and Drug Administration approved Symplicity Spyral (Medtronic) and Paradise Ultrasound Renal Denervation (Recor Medical) for use in the US.126,127 Both systems have received CE mark approval for the treatment of hypertension in Europe. RH might not be the optimal target population to validate the efficacy of renal denervation, but it does not negate the potential utility of this strategy in this context. Identifying individuals who are more likely to respond positively to renal denervation, such as those not included in RCTs because of advanced CKD, becomes crucial.128 In addition, performing such procedures demands operators with proficiency in renal interventions and specialised training. Centres offering renal denervation should possess the capability to address any potential complications.129
Conclusion
In the past decade, hypertension research has significantly advanced our understanding, particularly in defining RH. Distinguishing between tRH and aRH has become crucial, as these represent distinct forms of the disease with different management strategies and prognosis. Addressing aRH primarily involves promoting adherence to drug therapy and lifestyle recommendations. While tRH constitutes a smaller percentage of the hypertensive population, certain sub-groups, like those with diabetes or CKD, are at higher risk. These patients present with increased rates of CV and renal complications, warranting aggressive medical surveillance. Spironolactone emerges as the preferred fourth-line treatment for RH.
Ongoing research on novel drugs (NCT04277884) and non-pharmacological treatments including renal denervation might provide further therapeutic options in the coming years.