Heart failure (HF) and chronic kidney disease (CKD) often coexist, and the prevalence of both conditions is increasing. Forty-nine per cent of HF patients also have CKD, and an estimated 17–21% of CKD patients develop de novo HF.1,2 Each condition is independently associated with significant morbidity and mortality, and these effects are compounded by each other.3 This is due to the kidney’s interdependent and complex relationship with the heart in its control of salt, water and hormone homeostasis. Despite the many trials investigating pharmacological therapies for HF, there exists groups of patients who remain under-studied. This review will discuss age and sex differences in HF patients, as well as perhaps the most under-studied group of HF patients, those with advanced CKD.
Pathophysiology
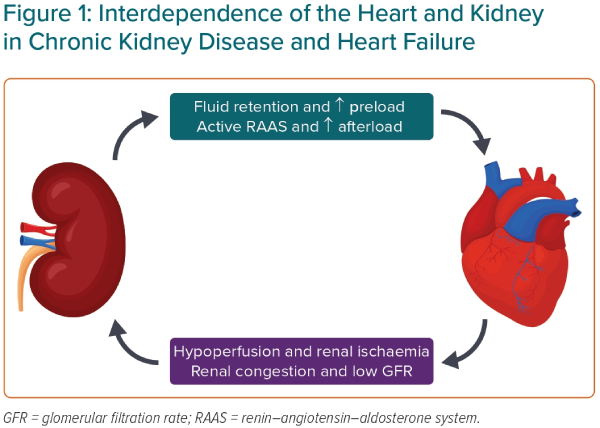
The reason CKD and HF so often coexist is because of their closely linked physiology in maintaining blood pressure and salt and water homeostasis (Figure 1). Dysfunction of one organ can cause the progressive dysfunction of the other. For example, in worsening CKD, the inability to excrete water due to decreased glomerular filtration causes an increased preload and can worsen HF.3 In HF, low cardiac output (particularly in HF with reduced ejection fraction [HFrEF]) and renal venous congestion cause renal hypoperfusion and the progression of CKD.3 In addition, renal hypoperfusion activates the renin–angiotensin–aldosterone system, which causes efferent arteriole constriction, increasing glomerular perfusion pressures, as well as fluid retention, which can exacerbate HF.
Heart Failure Therapies and Serum Creatinine
Serum creatinine often increases when initiating or uptitrating HF therapies, including angiotensin-converting enzyme inhibitors (ACEi), angiotensin receptor blockers (ARB), mineralocorticoid receptor antagonists (MRA) and angiotensin receptor–neprilysin inhibitors (ARNI). These drugs cause efferent arteriole dilation and decreased filtration pressures, accompanied by a decrease in estimated glomerular filtration rate (eGFR). A similar effect is seen during the initiation or uptitration of sodium–glucose cotransporter 2 (SGLT2) inhibitors (SGLT2i), but through a different pathophysiology.4 Inhibition of the SGLT2 causes less water and glucose to be reabsorbed in the proximal convoluted tubule, thus causing expansion of the distal convoluted tubule, resulting in constriction of the afferent arteriole to maintain glomerular perfusion pressure and a decrease in eGFR. Due to these changes in eGFR, clinicians may be deterred from initiating or uptitrating these therapies due to fears of worsening renal function, especially in patients with severe CKD. However, these dips in eGFR are not due to renal damage, but rather to changes in glomerular haemodynamics, and these therapies have been shown to slow the rate of decline of eGFR over time.5,6
Sex Differences
A recent Nature review found that women have a higher prevalence of non-dialysis-dependent CKD than men, but this is likely confounded by women living longer than men and from the overdiagnosis of CKD in women caused by the eGFR equations.7 In addition, men are more likely to have end-stage renal failure and tend to have a faster decline in kidney function, possibly attributed to unhealthier lifestyles and the effect of sex hormones.7 A systematic review and meta-analysis of HF patients found that in 30 papers studying the predictors of worsening renal function, the strongest predictors were baseline CKD (28 studies), hypertension (13 studies), diabetes (13 studies), advanced age (11 studies) and the use of diuretics (12 studies), whereas female sex was a predictor in four studies.1
With respect to cardiovascular disease risk factors, data from the US have shown there to be no sex differences in overall improvements in the control of hypertension, diabetes and dyslipidaemia, but women are more likely to have better-controlled hypertension and diabetes and less likely to have better-controlled dyslipidaemia compared with men.8 In addition, the effects of traditional risk factors, including overweight and obesity, hypertension and hyperlipidaemia, are similar between the sexes.9
Although the prevalence of cardiovascular disease (CVD) is higher in women, this may be due to the longer life expectancy and higher proportion of the elderly population being female.10 Mortality rates for CVD remain higher in men than in women.10 A recent systematic review found that among CKD patients, men had significantly worse CVD outcomes, including death, than women.11 Heart failure with preserved ejection fraction (HFpEF) is more common in women than in men. Clinical trials investigating ARNI (Paragon-HF), ARB (I-PRESERVE) and MRA (Top Cat) have shown unconvincing benefits for HFpEF.12–14 Interestingly, a substudy of the Paragon-HF trial showed that after stratifying by sex, sacubitril/valsartan (Entresto, Novartis) significantly reduced first and recurrent hospitalisations for HF and death from cardiovascular causes in women but not in men.15
More recently, the DELIVER, SOLOIST-WHF, and EMPEROR-Preserved trials showed that the SGLT2i dapagliflozin, sotagliflozin and empagliflozin, respectively, reduced the composite outcomes of hospitalisations and cardiovascular deaths, regardless of ejection fraction or diabetes status.16–18 Although many patients with HFpEF are on ACEi/ARB and beta-blockers regardless of the evidence from the aforementioned trials, the mainstay of management for HFpEF remains the management of risk factors, including hypertension and coronary artery disease.19 The lack of evidence-based pharmacological therapies for HFpEF is more likely to affect women than men. A summary of the pivotal HF trials from the past 2 decades shows the representation of women in modern studies (Supplementary Material Table 1). Naturally, studies of HFpEF tended to have a higher proportion of women than studies of HFrEF, as reflected by the epidemiology of HF. However, given that over half of all HF patients in the community are women, they are still under-represented in modern HF randomised control trials.19
Age Differences
With medical advances and increased access to healthcare, much of the world is experiencing an ageing population. As for many other conditions, the development of CKD and HF is associated with advanced age. From Supplementary Material Table 1, the mean age of participants in recent major HF trials is 68 years, with the most recent three trials of SGLT2i having a mean age of at least 70 years for each study.16–18 However, the point prevalence of HF increases with age up to and beyond 85 years.20,21 HF incidence is declining overall, but this decline is being driven by a lower HF incidence in patients aged 60–84 years, a very similar age range represented by major HF clinical trials.22 The incidence of HF in patients not represented in clinical trials (i.e. those younger than 55 years and older than 85 years) has remained stable or is, in fact, increasing.22 Interestingly, the very elderly population of HF patients may have a different prevalence of risk factors than younger patients. For example, one study showed that HF patients older than 85 years are more likely to be women and have HFpEF, and have a lower prevalence of CVD risk factors, including diabetes, hypertension and ischaemic heart disease; however, the prevalence of non-cardiovascular comorbidities continued to increase linearly with age.23
Although modern trials do not have criteria explicitly excluding the elderly, other common exclusion criteria, such as hyperkalaemia, anaemia, severe CKD, active or recent malignancy and other clinically significant coexisting conditions, such as frailty, are likely to limit their trial participation and will inevitably exclude older HF patients.24 Although older HF patients are less represented in clinical trials than younger patients, another large group of patients who are even less well represented are those with advanced CKD. The under-representation of advanced CKD patients is also a likely contributor to the under-representation of older patients in clinical trials.
Advanced CKD
Studies of the pharmacological therapies for HF (ACEi/ARB, ARNI, SGLT2i and MRA) are well researched for patients with an eGFR of 30 ml/min/1.73 m2 or above, but there is a paucity of evidence for patients with advanced CKD (Stages 4 and 5). There is moderately strong evidence for ACEi and ARB in CKD Stage 5 patients on dialysis, but weak evidence for CKD stage 5 patients not on dialysis, as reflected by Kidney Disease: Improving Global Outcomes (KDIGO) guidelines.25 However, the recent STOP-ACE showed that CKD Stage 4/5 patients who continued their ACEi/ARB had the same number of adverse events and were less likely to develop end-stage kidney disease or need renal replacement therapy compared with patients whose ACEi/ARB was discontinued.26
The EMPHASIS-HF and Top Cat trials showed the efficacy of MRA in HF patients with eGFR >30 ml/min/1.73 m2, but MRA are not currently recommended for advanced CKD because safety and efficacy data are lacking.13,27 There is a relatively strong body of evidence for β-blockers in CKD-HF patients. Bisoprolol has been shown efficacious and safe in HF patients with serum creatinine <300 µmol/l (CIBIS-II trial) and carvedilol has been shown to reduce morbidity and mortality in HFrEF patients with CKD Stage 5 requiring dialysis.28,29
Although only PARADIGM-HF showed a statistically significant benefit of sacubitril/valsartan in reducing death and HF admissions, both the PARADIGM-HF and Paragon-HF trials showed sacubitril/valsartan to be safe in HF patients, regardless of ejection fraction, with an eGFR of ≥30 ml/min/1.73 m2.14,30 Although no study has shown ARNI to be safe in CKD Stage 4 or 5 for HF patients, the UK HARP-III trial showed sacubitril/valsartan to be as safe as irbesartan in patients without HF and with an eGFR as low as 20 ml/min/1.73 m2.31 SGLT2i have been proven safe and efficacious in patients with CKD and an eGFR of 25–75 ml/min/1.73 m2 (DAPA-CKD trial), as well as in patients with HF and an eGFR of ≥20 ml/min/1.73 m2 (EMPEROR-Preserved and EMPEROR-Reduced trials); however, no trials have investigated their efficacy and safety when initiated in more advanced CKD.16,32
A potential reason for the exclusion of patients with severe CKD from trials may be fear of the initial drop in eGFR and hyperkalaemia seen when initiating or uptitrating many of these therapies, including ACEi/ARB and SGLT2i. However, as previously discussed, these drops in eGFR are related to haemodynamic changes in the glomerulus and are not indicative of kidney damage.19 These drops in kidney function will recover and, over the years, these medications will cause a slower rate of decline in kidney function.19,25
Conclusion
Although the elderly and women are underrepresented groups in research of CKD and HF, those with advanced CKD (Stages 4 and 5) are perhaps even more in need of prospective clinical trials to provide an evidence base for HF therapies in this growing population of patients.