Pregnancy in women with cardiac disease is associated with life-threatening complications. Although there has been progress in the field, cardiac disease in pregnancy remains among the leading causes of maternal and neonatal mortality and morbidity.1–3 Many studies have shown correlations between pregnancy-related haemodynamic changes and cardiac events.4–7 However, few studies have investigated factors related to adverse outcomes in the context of resource-limited settings. In addition, the condition has been poorly studied in most developing regions, leading to a poor understanding of the effects of cardiac disease on pregnancy outcomes among both clinicians and affected women of reproductive age. The aim of this study was to identify factors related to adverse maternal and neonatal outcomes in pregnant women with cardiac disease in low-resource settings.
Methods
This hospital-based longitudinal case series study was conducted between October 2016 and October 2018 in Kenya. The inclusion criteria were abnormal echocardiography and ECG findings in pregnant women and/or women in the postpartum period, as reviewed by an independent cardiologist.
The study was approved on 27 September 2016 by the Moi University, Moi Teaching and Referral Hospital Institutional Research and Ethics Committee (IREC; Approval no. FAN: IREC1756). All women enrolled in the study provided written informed consent.
Definitions of Abnormal Echocardiogram and Electrocardiogram Findings
Abnormal echocardiogram findings were defined as follows:
- left ventricular (LV) dysfunction with an ejection fraction <55%;
- diastolic dysfunction (E/A ratio <1 and a diastolic time [DT] >200 ms, and the presence of LV hypertrophy [LVH] in patients with Grade I diastolic dysfunction);
- right ventricular hypertrophy (RVH), with subcostal wall thickness ≥6 mm (classified as mild, moderate or severe);
- wall motion abnormalities (hypokinesia, akinesia or dyskinesia); or
- valvular abnormalities.
Abnormal ECG findings were defined as the presence of arrhythmias and ST and QRS segment abnormalities.
Data Collection
Patients with a confirmed diagnosis of cardiac disease were interviewed and the following data were collected: maternal age, education level, marital status, occupation, residence location, health insurance coverage, parity, BMI, tobacco and alcohol use, type of cardiac disease, New York Heart Association (NYHA) functional class and modified WHO (mWHO) risk index, comorbidities (diabetes, chronic hypertension, hyperthyroid disease, renal disease, coagulopathy, HIV/AIDS, venous thromboembolism, anaemia, malnutrition and mental illness), preconception care, cardiac disease prior to current pregnancy, treatment prior or during pregnancy, prior surgical cardiac intervention, mode of admission, maternal antenatal care history, level of care facility attended according to the national health system, gestational age at the time of enrolment and number of foetuses.
Foetal wellbeing was assessed using obstetric ultrasound and was defined as a normal biophysical profile (foetal heart rate, breathing movements, body movements, muscle tone and amniotic fluid index) or the absence of foetal abnormalities. For pregnancy dating, if the difference between the last menstrual period and the ultrasound findings was >7 days, the ultrasound assessment was used as the reference. In addition, details regarding the mode and place of delivery were recorded.
The severity of cardiac disease was lesion specific and included mitral stenosis (MS), mitral regurgitation (MR), aortic stenosis, aortic regurgitation (AR), tricuspid stenosis, tricuspid regurgitation and pulmonary arterial hypertension. These lesions were classified as mild, moderate or severe. The severity of MS was defined as follows:
- mild: mitral valve area (MVA) >1.5 cm2 or a mean gradient <6 mmHg;
- moderate: MVA 1.0–1.5 cm2 or a mean gradient 6–12 mmHg; and
- severe: MVA <1.0 cm2 or a mean gradient >12 mmHg.
Heart valvular diseases were defined according to the European Association of Echocardiography and American Society of Echocardiography recommendations for the echocardiographic assessment of valve lesions.8 Regurgitant valve lesions, including MR, AR, pulmonary hypertension and tricuspid regurgitation , were quantified on the basis of a visual inspection of echocardiographic findings.
Congenital heart disease was classified according to the size of the lesion rather than its anatomical position; lesions were classified as small, moderate or large. Cardiomyopathies were classified as hypertrophic or dilated. The type of cardiac disease was defined as rheumatic heart disease (RHD), congenital heart disease or cardiomyopathy. NYHA functional classes were used to define patients as asymptomatic (NYHA Class I) or symptomatic (NYHA Classes II, III and IV). In addition, the mWHO risk tool was used in to divide patients into groups, as follows9:
- low risk (mWHO Category I): no detectable increased risk of maternal mortality and no or a mild increased risk for morbidity;
- medium risk (mWHO Category II): minor increased of maternal mortality or moderate morbidity;
- high risk (mWHO Categories II–III and III): significant increased risk of maternal mortality or severe morbidity; and
- extremely high risk (mWHO Category IV): extremely high risk of maternal mortality or severe morbidity.
Details of mWHO risk categories are provided in Supplementary Material Box 1.
Adverse maternal outcomes (prespecified adverse outcomes) were defined as the occurrence of cardiac and obstetric events. Cardiac events included heart failure (HF), pulmonary oedema, arrhythmias, cardiac arrest, endocarditis and thromboembolic events. Chest radiography was used to confirm the clinical diagnosis of pulmonary oedema. Obstetric events consisted of preterm labour and preterm delivery, antepartum or postpartum haemorrhage, depression, stillbirth, miscarriage, therapeutic abortion, admission to the intensive care unit (ICU) and maternal death, all of which were recorded according to gestation period.
The primary outcome of the study was the occurrence of one or more adverse events, whereas secondary outcomes were successful delivery or recovery or improvement within 6 weeks postpartum.
Statistical Analysis
Data were analysed using IBM SPSS version 20.0. (IBM). Categorical data are described as frequencies and percentages, whereas continuous data are described as interquartile range, mean ± SD or median values. The Kolmogorov–Smirnov test was used to verify the normal distribution of quantitative data. Significance was set at two-tailed p<0.05. The chi-squared test was used to compare categorical between groups based on outcomes. Fisher’s exact test or Monte Carlo correction was used for chi-squared analysis when more than 20% of the cells had an expected count <5. The ORs and 95% CIs for maternal adverse outcomes were calculated using logistic regression.
Results
There were 91 pregnant women with cardiac disease recruited to the study. Of those, 98.9% had complete follow-up data, and one was lost to follow-up. The socio-demographic characteristics of the women are summarised in Supplementary Material Table 1. The distribution of cardiac diseases and maternal clinical characteristics are summarised in Supplementary Material Table 2.
Maternal Outcomes
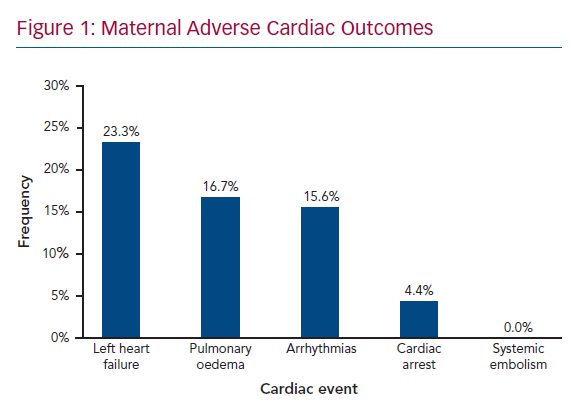
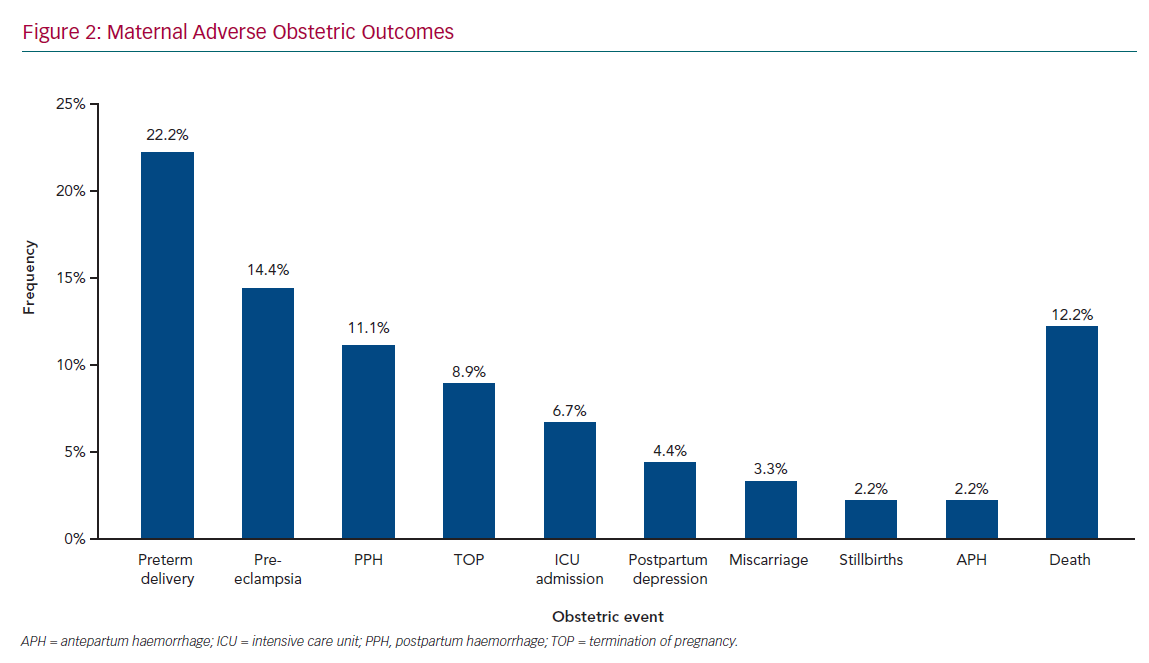
Figure 1 shows maternal cardiac adverse outcomes. Maternal cardiac events occurred in 60% of women, with the most common complication being HF, which accounted for 23.3% of all cardiac events, followed by pulmonary oedema (16.7%) and arrhythmias (15.6%). Maternal cardiac arrest was the least common adverse cardiac event, occurring in 4.4% of women. HF and arrhythmias predominantly occurred in the second trimester, between 14 and 28 weeks’ gestation, in 25.9% and 14.8% of women, respectively. Pulmonary oedema was also frequent in the postpartum period (18.5%). Obstetric adverse outcomes (Figure 2) occurred in 75.6% of women, with preterm delivery being the most common (22.2%), followed by pre-eclampsia (14.4%) and postpartum haemorrhage (11.1%), termination of pregnancy (8.9%), postpartum depression (4.4%), miscarriage (3.3%), and stillbirths (2.2%). These events contributed to 16.2% of all maternal deaths; 8.8% of adverse events required admission to the ICU. Maternal cardiac and obstetric events according to gestation period are summarised in Supplementary Material Table 3. Supplementary Material Figure 1 shows maternal deaths according to type of cardiac disease: 12.2% of patients died as a result of their cardiac disease. RHD was the leading cause of maternal death, responsible for eight deaths (73% of all deaths, but 10% of all women with RHD).
Trends for Factors Associated With Adverse Outcomes
Socio-demographic factors such as maternal age, marital status and occupation were not significantly associated with adverse outcomes. However, a low education level (primary), which was not significantly associated with adverse outcomes (p=0.126), increased the odds of adverse outcomes 3.3-fold after adjustment for confounders (95% CI [1.0–11]; p=0.049). In addition, residence location was significantly associated with adverse outcomes (p=0.009), with 100% of women from rural areas experiencing some form of adverse outcome. However, adjustment for confounders, residence location was not associated with an increased risk of adverse outcomes (Supplementary Material Table 4).
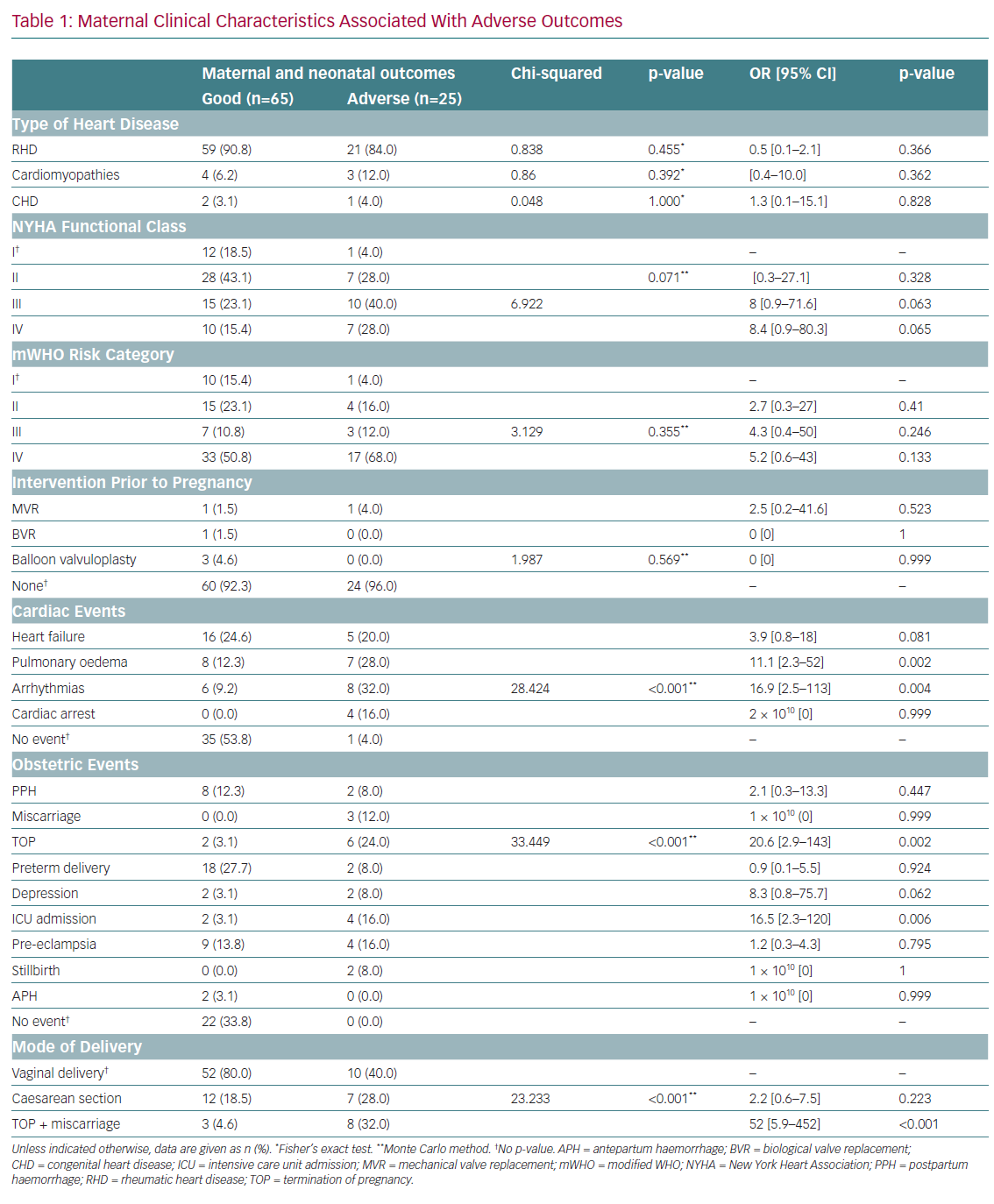
The type of cardiac disease, NYHA functional class, mWHO risk category and prior cardiac surgery intervention were not significantly associated with adverse outcomes (Table 1). However, any cardiac event was significantly associated with adverse maternal outcomes (p≤0.001). After adjustment for confounders, pulmonary oedema and arrhythmias were associated with significant 11- and 17-fold increases, respectively, in the odds of adverse outcomes.
Obstetric adverse events were significantly associated with adverse outcomes (p≤0.001). After adjustment for confounders, therapeutic abortion and late maternal ICU admission were associated with significant 20.6-fold (95% CI [2.9–143]) and 16.5-fold (95% CI [2.3–120]) increases in the risk of adverse outcomes, respectively. Mode of delivery was also significantly associated with adverse outcomes (p≤0.001). After adjustment for confounders, only therapeutic abortion remained significant and was associated with a 52-fold increase in the odds of adverse outcomes (95% CI [5.9–452]; p≤0.001).
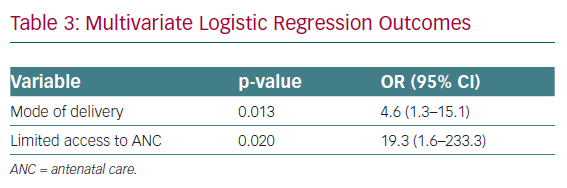
Limited access to quality antenatal care, parity, place of delivery and the level of the facility that provided care were significantly associated with adverse outcomes (Supplementary Material Table 5). After adjustment for confounders, delivery at home increased the risk of adverse outcomes approximately 23-fold (95% CI [2.3–224]; p=0.007). Mode of delivery and limited access to quality care were significantly associated with maternal adverse outcomes, increasing the risk fourfold and 19-fold, respectively (Supplementary Material Table 5).
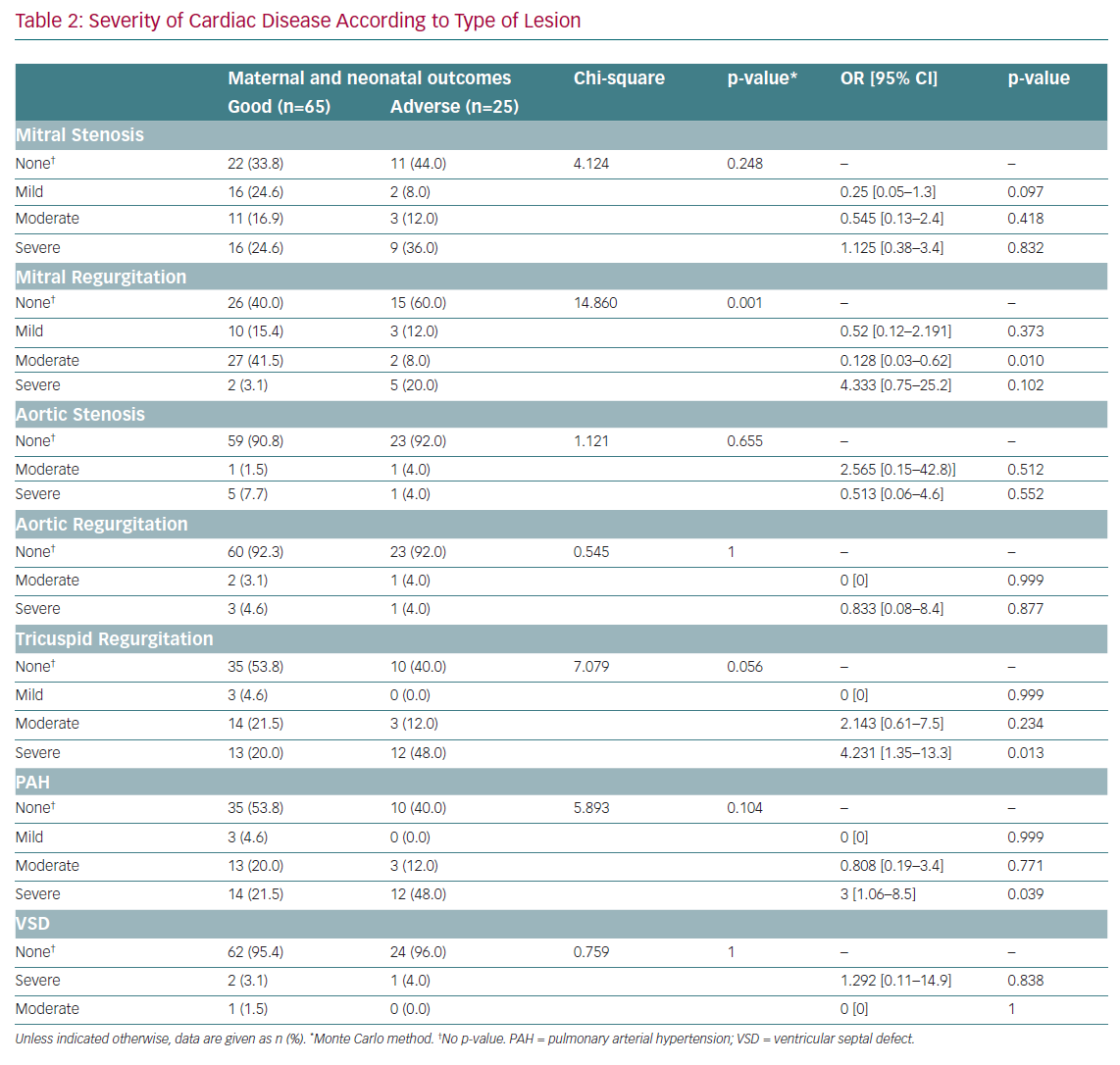
MR (mild, moderate and severe) was significantly associated with adverse outcomes (p=0.001; Table 2). After adjustment for confounders, moderate MR was found to be protective against adverse outcomes (OR 0.128; 95% CI [0.03–062]; p=0.010). MS, aortic stenosis, AR, tricuspid disease and ventricular septal defects were not significantly associated with maternal adverse outcomes. After adjustment for confounders, severe tricuspid disease and severe pulmonary arterial hypertension were associated with 4.2-fold (95% CI [1.3–13.3]) and threefold (95% CI [1.1–8.5]) increased risks of maternal adverse outcomes, respectively.
Discussion
The rate of cardiac adverse events was higher in the present study than in previous studies.6,7 This study had similar findings to the Cardiac Disease in Pregnancy (CARPREG) II study, where most cardiac complications occurred during the antepartum period, followed by the postpartum period, and cardiac complications during the intrapartum being the least frequent.7 This is not surprising because more than half the women in the present study who were diagnosed with cardiac disease during pregnancy were not receiving treatment. The delay in the decision to seek care could be the main reason for the higher rate of cardiac events in this study. HF, the leading cardiac complication, occurred most often in the second trimester. This is congruent with findings of previous studies regarding the gradual haemodynamic changes in pregnancy and the occurrence of HF in pregnant women with cardiac disease.10–14 However, the finding of the present study is in contrast with those of the CARPREG II Study, in which HF was predominantly reported during the third trimester and postpartum period.7 However, in contrast with the CARPREG II study, the present study considered HF and pulmonary oedema as two different cardiac complications. Among participants who experienced pulmonary oedema during the antepartum period, one who had severe mitral stenosis and aortic stenosis developed fatal acute pulmonary oedema following administration of 12 mg of dexamethasone for foetal lung maturity. Corticosteroid-induced pulmonary oedema in patients with rheumatic heart disease have not been investigated. However, fluid retention, known as one of the cardiovascular effects of corticosteroids, could explain the occurrence of acute adverse event. Therefore, if the risk of corticosteroid therapy to the mother outweigh the benefit to the foetus, it is wise for the clinician to withhold the treatment. Instead, preterm infant should benefit surfactant administration after delivery. Cardiac arrhythmias frequently occurred during the antepartum period, AF being the predominant arrhythmia. AF frequently occurred in the group of patients who did not have arrhythmias at time of admission but developed complications during termination of pregnancy (TOP). Misoprostol, a synthetic prostaglandin (PG) E1, was the drug used for TOP in all patients who underwent therapeutic abortion.
The rate of obstetric adverse events in the present study was sevenfold times higher than that reported in previous studies.6,7 An incidental finding of cardiac arrhythmias was reported among women who underwent therapeutic abortion with PGE1 from mid-pregnancy. The observed adverse outcomes ranged from arrhythmias to death. To date, no data are available regarding the cardiovascular effects of misoprostol, a drug widely used in resource-limited settings for the induction of labour and TOP. However, the European Society of Cardiology (ESC) has reported theoretical risks of coronary vasospasm and arrhythmias as side effects of PGE1.5 We acknowledge the recent recommendation in the ESC guidelines suggesting the use of 100 µg PGE1 for the termination of pregnancy up to 9 weeks or surgical abortion beyond 9 weeks.5
In the present study, most patients who underwent TOP because of their risk index category received two to three doses of ≥200 µg misoprostol at 4-hour intervals, depending on how the individual patient responded to the initial dose. Most women who died after TOP died because of similar complications, including pulmonary oedema (confirmed by autopsy) and AF. They also had similar characteristics, including severe MS, severe pulmonary hypertension and tricuspid disease. This incidental finding needs further investigation in a larger sample size. However, carrying out such study poses ethics dilemma for approval. In addition, the authors critically examined the recommendation to initiate TOP in mid-pregnancy based on the mWHO risk category index and concluded that the recommendation lacked expert consensus. Moreover, previous studies acknowledged the limitations of predictors and risk scores when making decisions and recommended additional alternative management options, including obtaining expert opinion, hospitalisation of extremely high-risk patients up to delivery or exclusive care in a specialist unit.15–17 Furthermore, percutaneous balloon mitral commissurotomy in the second trimester is currently recommended for mothers with severe valvular heart lesions who do not respond to medical treatment.18,19 Therefore, we recommend TOP in only women presenting with refractory HF. At term or nearly at term, mechanical Foley catheter induction of labour is preferred. However, low-dose oxytocin has been safely used in low-risk patients.20
Most ICU admissions in the present study occurred during the postpartum period. However, most of these patients had poor outcomes. A lack of health insurance was a major contributor to poor outcomes due to limited access to ICUs in private hospitals when public hospital ICUs are full. The authors acknowledge the expert consensus regarding timely ICU admission for labour and delivery of high-risk and extremely high-risk patients.5,16,17 However, the cost of ICU care and a lack of adequate infrastructure and trained staff are major obstacles in resource-limited settings.21–23 In this study, most of the high- and extremely high-risk patients were managed and delivered at the cardiac care unit (CCU), and only one mother died out of 22 deliveries at the CCU. Therefore, the CCU is a good alternative to the ICU for monitoring high- and extremely high-risk pregnant women in labour or after delivery.
Demographic factors and adverse outcomes in pregnant women with cardiac disease have been investigated across the world. For example, in the CARPREG II study maternal age <18 and >35 years was reported to be associated with adverse outcomes.7 This is in contrast with the findings in the present study because, in the context of developing countries, age alone cannot explain the occurrence of adverse outcomes. Adverse outcomes are the product of an interplay of multiple factors, including poor health literacy, disease severity, poor health-seeking behaviour and poverty. In addition, previous studies have described the association between maternal education level and adverse outcomes.24–26 Similarly, a low maternal education level increased the odds of maternal adverse outcomes, which can clearly be explained by poor health literacy, and a low socioeconomic level translates to poverty, both of which affect the decision to seek care. Residence location was associated with maternal adverse outcomes, especially in rural dwellers, but was not found to increase the risk of adverse outcomes. This can be explained by limited timely access to tertiary hospital care and poverty. The Registry of Pregnancy and Cardiac Disease (ROPAC), recently recognised limited access to specialised care as one of the causes of poor outcomes in developing areas.6 In addition, adverse outcomes likely occur because of the trifecta of a delay in seeking care, reaching the right facility and receiving appropriate treatment.27
RHD was the predominant cardiac disease in pregnancy, and accounted for most adverse outcomes. This is congruent with previous studies that identified RHD as the leading cause of death in developing countries. Moreover, Diao et al. reported that up to 34% of maternal deaths were attributable to rheumatic disease.³ In the present study, every 10th pregnant woman admitted with RHD died during pregnancy. This is evidence that mortality due to RHD is still far from controlled in most developing areas. Almost all rheumatic disease-related deaths or observed adverse outcomes were reported in women with severe MS and/or complex heart disease, including severe pulmonary hypertension and severe tricuspid disease. Pieper and Hoendermis, along with other investigators, reported similar findings in which valvular disease, especially MS, was the leading cause of secondary pulmonary hypertension, which is associated with high mortality in affected pregnant women.9,28
NYHA functional class was not associated with adverse outcomes. This contrasts with results from previous studies in which advanced cardiac functional class was repeatedly reported to predict poor maternal and foetal outcomes.6,7 These conflicting results are due primarily to differences in the study populations. The mWHO risk category was not associated with adverse outcomes. van Hagen et al., from the ROPAC, reported a similar finding that was particularly evident in data from developing countries where the mWHO risk category tool did not perform well.6 This may be related to the interaction of multiple factors, including socio-demographic and clinical factors, especially the lack of universal access to state-of-the-art care by multidisciplinary expert teams; access to these teams would minimise the effects of practice differences in the management of such high-risk patients. In addition, Balci et al. found another weakness in the mWHO risk classification: expert knowledge is sometimes more important than individual risk assessment.29 Such knowledge or expertise is lacking in resource-limited settings.
Surgical intervention prior to pregnancy was not found to be associated with adverse outcomes. This could be related to the low number of patients who underwent surgical correction before pregnancy. The original CARPREG study risk prediction tool did not include this variable; however, in the CARPREG II study, prior cardiac intervention for placement of a mechanical prosthesis increased the rate of adverse outcomes.7 These adverse outcomes are primarily teratogenic effects and abortion associated with anticoagulation, especially warfarin, or thromboembolic events associated with mechanical valve prosthesis.19 This study recognises that surgical cardiac interventions use new technologies that are out of reach of the majority of patients in developing countries. To date, Kenya is among the few countries in sub-Saharan Africa with the capability to perform heart surgery, with five facilities across the country. Although surgical treatment is available in locations relatively accessible to patients, the low socioeconomic level of most patients is a major obstacle to obtaining treatment for cardiac diseases. Notably, not all patients are eligible for cardiac intervention. In their study, van Hagen et al. found that percutaneous balloon commissurotomy, which is also performed during the second trimester of pregnancy, significantly improved maternal and perinatal outcomes.18
The place and mode of delivery were associated with adverse outcomes. Infants born at home died within 3 days of birth, and their mothers were likely to be admitted to the ICU. The plausible explanation of complications related to home births is that the delivery can be assisted by an unskilled person or in an unsafe environment with limited access to essential newborn care or management of cardiac events that may occur after delivery.
In their retrospective study, Titaley et al. reported both maternal and neonatal complications were associated with home birth, even if the deliveries were conducted by trained nurses.30 Caesarean delivery was significantly associated with the risk of adverse outcomes. This is not surprising because the use of anaesthetic agents (general or regional anaesthesia) is known to be an important factor triggering cardiac events, especially cardiac arrest.9,31,32 To reduce anaesthesia-related mortality and morbidity in women with cardiac disease and based on the cardiovascular effects of each drug, expert application of regional anaesthesia is the preferred method, although it has minimal benefits.9,31 Moreover, women with pulmonary hypertension were found to have an increased risk of developing cardiac events with general anaesthesia, whereas general anaesthesia appeared to be the safest means of successful delivery in women with aortic stenosis.9,31 However, evidence has shown that caesarean delivery confers no advantage for maternal and neonatal outcomes.32–34 Indeed, caesarean delivery should only be performed for obstetric indications, defined as inability for the mother to achieve vaginal delivery at specific-time.
Conclusion
Maternal adverse outcomes in women with cardiac disease are multifactorial in origin and include clinical and non-clinical factors. Timely access to comprehensive care and expertise regarding the management of cardiac diseases during pregnancy can contribute significantly to reducing maternal mortality and morbidity due to cardiac diseases.
Click here to view Supplementary Material.