There has been rapid progress in percutaneous coronary intervention (PCI) in recent years in terms of both technology and adjunct therapy. Revascularisation is indicated in specific subgroups of patients with ischaemic heart disease aiming to improve symptoms and outcomes. Although PCI treats local stenosis by increasing the left main (LM) in the dilated and stented area, coronary artery bypass grafting (CABG) improves blood flow to the jeopardised myocardium, protecting the distal myocardial territory from future ischaemic events due to proximal lesions.1 Similar survival results have been reported for revascularisation with both techniques.2–4 However, specific subsets of patients may benefit more with CABG or with PCI. This systematic review focuses on the selection of the revascularisation strategy for left main coronary artery disease (LMCAD) and aims to help cardiologists to make this complex decision.
Current clinical guidelines recommend revascularisation for all patients with significant (≥50%) LMCA lesions regardless of symptoms and/or inducible ischaemia.5
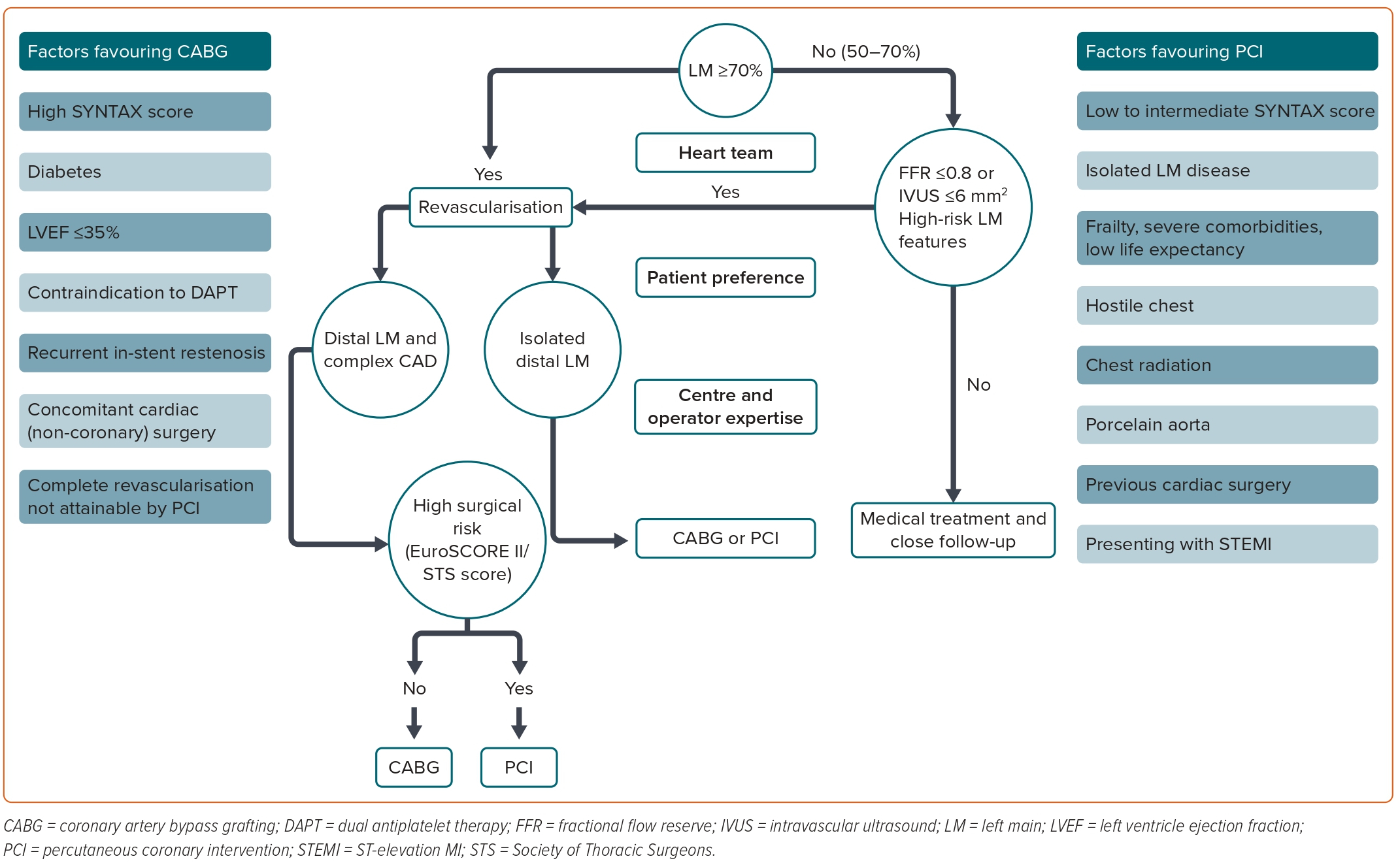
Depending on patient clinical characteristics and the complexity of the coronary anatomy, CABG or PCI would be indicated. PCI outcomes depend on the location of LM stenosis, the presence of bifurcation involvement and the overall coronary atherosclerotic burden. There are well-defined angiographic features that increase coronary artery disease (CAD) complexity and well-defined patient characteristics that make one of the revascularisation strategies the first choice.1 Considering these relevant findings, as well as the experience of both the centre and the operator and patient preferences, decisions should be individualised.
Indications for PCI or CABG
The 2021 American College of Cardiology/American Heart Association/Society for Cardiovascular Angiography and Interventions (ACC/AHA/SCAI) Guideline for Coronary Artery Revascularization highlights different situations in which PCI or CABG would be preferred.1
Complex Coronary Artery Disease
In patients with LMCAD and high anatomical complexity, CABG is the first choice to improve survival.2–6 Angiographic features contributing to increased LMCAD complexity are distal and bifurcation lesions (PCI is more feasible for ostial or mid-shaft LM stenosis), significant calcifications, severe tortuosity, a thrombotic lesion and/or long stenosis.1
An objective assessment to determine the anatomical coronary complexity can be achieved using the SYNTAX score. This tool was prospectively developed from the SYNTAX trial results to help cardiologists decide which could be the most appropriate revascularisation strategy.7 The SYNTAX score is a long-term independent predictor of major adverse cardiac events (MACE) and death that has been validated in studies of patients treated with PCI, but not CABG.7,8 The SYNTAX score II, which has been validated in randomised controlled trials (RCTs) and registries, combines the anatomy-based SYNTAX score with clinical prognostic variables with the aim of estimating mortality and, therefore, improving the decision in the choice between PCI and CABG for patients with multivessel CAD. The clinical variables in the SYNTAX score II include the presence of unprotected LMCAD, female sex, chronic pulmonary disease, age and ejection fraction.1 This tool will be superseded by the Logistic Clinical Syntax Score (2020), also based on coronary anatomy and comorbidities, in which PCI predicts all-cause mortality at 2 years in all-comers PCI.9 Although clinical guidelines base recommendations regarding the revascularisation approach on the SYNTAX score, it is underused in clinical practice because of some limitations, such as its complexity and being a time-consuming tool that requires high interobserver variability.
The complete revascularisation expected, with the aim of minimising the residual ischaemia region, is another issue to be considered when choosing the revascularisation strategy. Usually, complete revascularisation is more likely with CABG, and revascularisation has been associated with a better prognosis, as reported in the nuclear substudy of the COURAGE trial, which found a benefit in reducing the risk of death and MI by reducing residual stress-induced ischaemia from >10% of the myocardium to ≥5%.10
However, recent results of the REVIVED trial, with no survival benefit in revascularisation with PCI versus optimal medical therapy in patients with severe ischaemic left ventricular systolic dysfunction, question the hypothetical benefit of achieving complete revascularisation.11 These results are not explained by incomplete revascularisation, because complete revascularisation was achieved in 71% of those in the PCI group, a relatively high percentage considering that only coronary lesions with viable myocardium were revascularised. However, although all patients included had demonstrable viability in at least four dysfunctional myocardial segments amenable to revascularisation with PCI, no physiological assessment of lesions was conducted, and 66% of patients in the PCI group were free of angina.11 Therefore, a certain proportion of lesions underwent PCI that was not indicated. As a hypothetical explanation for this negative result, the authors pointed out the fewer number of primary outcome events recorded than estimated for the trial to have an 85% power to address the primary hypothesis.11 For this reason, the statistical power of the study could be slightly compromised.
Patients with Diabetes and Multivessel Coronary Artery Disease
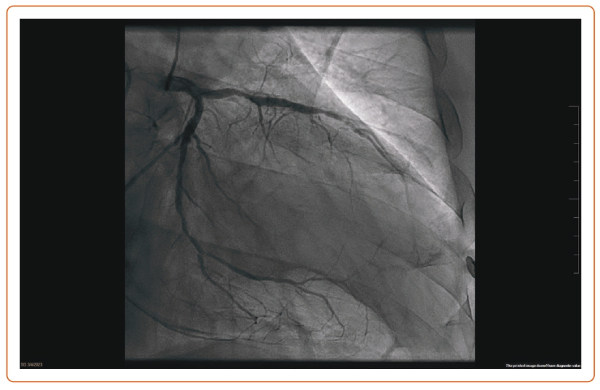
Diabetes is associated with a two- to fourfold heart disease mortality risk, and a more diffuse and severe atherosclerosis involving the microcirculation and the small coronary vessels.1 After PCI, diabetic patients with multivessel CAD need a higher rate of long-term revascularisation and present a higher mortality rate at the 5-year follow-up than patients treated with CABG.6 This survival benefit of CABG is present 2 years after the revascularisation but is attenuated beyond 8 years.6 Remarkably, there are no published RCTs comparing PCI and CABG in diabetic patients with LMCAD. Figure 1 shows an example of diffuse CAD in a patient with type 2 diabetes.
Patients with Previous CABG
Both PCI and redo CABG in patients with previous CABG are associated with higher rates of revascularisation failure, complications and worse outcomes.12 Furthermore, no RCTs have compared revascularisation with either PCI or CABG versus medical therapy in patients with previous CABG. Availability of the left internal mammary artery (LIMA) for grafting, complexity of the coronary anatomy, patient preferences, clinical features and comorbidities are some of the factors to be considered by the heart team.13 However, considering the increased risk during CABG with higher rates of in-hospital death and stroke, PCI is the usual revascularisation strategy in patients with previous CABG.13
Dual Antiplatelet Therapy Compliance and Safety
Stent thrombosis causes high morbidity and mortality.14 Therefore, comorbidities increasing the haemorrhagic risk, which may cause dual antiplatelet therapy (DAPT) discontinuation, as well as the patient’s socioeconomic status (because patients with economic difficulties are more likely not to adhere to antiplatelet treatment and could, therefore, present a higher risk of stent thrombosis) and lifestyle, should be considered because these may influence compliance and safety. Therefore, the 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization considers it reasonable to choose CABG instead of PCI in patients with multivessel CAD eligible for treatment with both strategies who are unable to receive, tolerate or comply with DAPT.15 However, the appropriate duration of DAPT in patients at high risk of bleeding after PCI with a drug-eluting stent (DES) is unclear, and there is compelling evidence supporting the safety and efficacy of abbreviated DAPT up to 1–3 months, especially in patients with stable angina.16 Furthermore, patients deemed to be at high bleeding risk frequently present a high surgical risk. Although it is important to assess bleeding risk, it is rarely a decisive factor in the revascularisation strategy.
Comparative RCTs and Meta-analyses of CABG versus PCI
Multiple RCTs and meta-analyses have compared the effectiveness and safety of CABG versus PCI in patients with LMCAD, showing no significant survival differences and therefore not answering this endless debate.2,6,16–19 The present systematic review is focused on the results of the four most relevant trials, namely the SYNTAX trial, the PRECOMBAT trial, the EXCEL trial and the NOBLE trial, which are summarised in Table 1.2,6,16–19
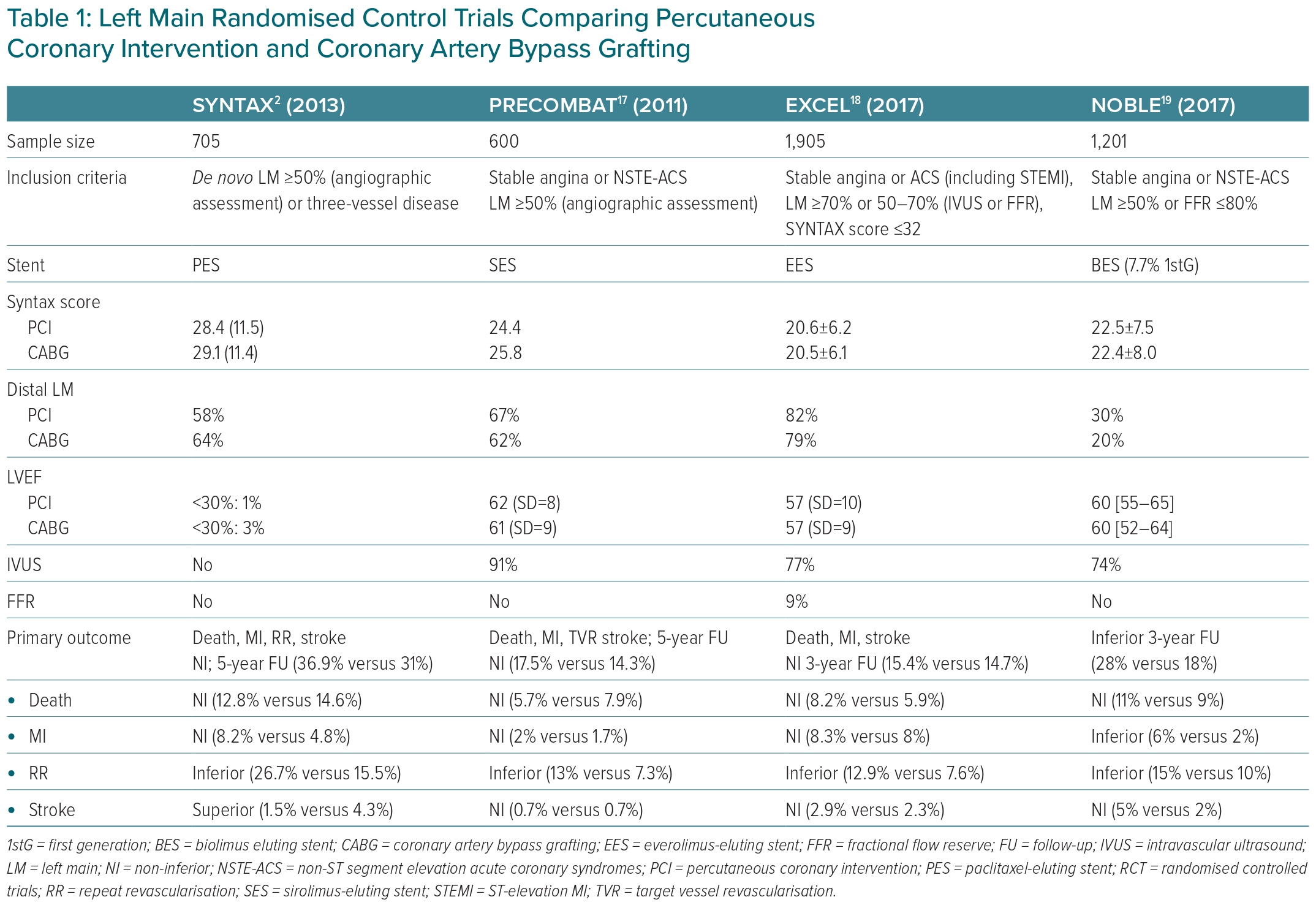
High heterogeneity in the inclusion criteria, primary endpoint definitions, length of follow-up and some other important confounding factors, such as different types of stents, explains the discordant results of these RCTs. For example, LM significant stenosis was defined as stenosis >50%, but in the EXCEL trial, patients with lesions between 50% and 70% were only included if stenosis was haemodynamically significant after functional assessment with fractional flow reserve (FFR; used in 9%) and/or intravascular ultrasound (IVUS; used in 77%). The NOBLE trial included patients with a ≥50% LM stenosis and/or a pressure guidewire assessment FFR ≤0.80 and no more than three coronary complex lesions.18 Moreover, in the EXCEL and NOBLE trials an IVUS-guided PCI strategy was recommended.20 Recent data show the prognostic benefit of intracoronary imaging, lowering the target vessel failure and stent thrombosis rates at 3 years.21,22 The patients included in these four trials comparing PCI plus DES (including first-generation DES) with CABG (predominantly with a LIMA graft) had a lower prevalence of diabetes (15–30%) compared with other PCI studies, most had a preserved ejection fraction and 60–80% had distal LM stenosis.2,17–19 First-generation DES, compared with new-generation DES, are associated with worse long-term clinical outcomes.23
The SYNTAX trial enrolled patients with LMCAD and/or multivessel CAD.2,6 In all, 705 patients with LM stenosis (overall mean SYNTAX score 30) were randomised to either CABG or PCI with the first-generation paclitaxel-eluting stent TAXUS Express. No differences were observed between the two groups at 1 or 5 years in the primary composite outcome of all-cause death, cardiac death and MI. Although CABG was associated with higher stroke rates, the PCI group had higher rates of repeat revascularisation. A survival benefit was only observed in patients with three-vessel disease treated with CABG. Interestingly, in patients with intermediate (23–32) or high SYNTAX (≥33) scores, the rate of major adverse cardiac and cerebrovascular events (MACCE) was significantly higher in the PCI group at 5 years follow-up, but not at 10 years, suggesting inconsistent results.
The remaining three big trials excluded patients with complex CAD. In the PRECOMBAT trial, 600 patients with LMCAD and a mean SYNTAX score of 25 were randomised to either PCI (with sirolimus-eluting stents) or CABG (93.6% with LIMA to the left anterior descending artery). PCI was non-inferior in mortality, MI, stroke and revascularisation at 1 and 5 years. IVUS was used in 91.2% of the PCI group. No differences were observed in the stroke rate and ischaemia-driven revascularisation was more frequent in the PCI than the CABG groups (11.4% versus 5.5%). In subgroup analyses, the only significant difference was observed in patients with LM and three-vessel disease, favouring CABG. Importantly, no significant difference between the two groups was seen in assessment of the SYNTAX score.17
In the EXCEL trial, patients with LMCAD and a low or intermediate (≤32) SYNTAX score were randomised to undergo PCI (with an everolimus-eluting stent) or CABG. Although the trial included patients with a low/intermediate SYNTAX score, 80% of patients had distal LMCAD. Although there was no significant difference between the two groups in the primary composite outcome of death, stroke or MI at 3 or 5 years, the results of the secondary composite outcome of death, stroke, MI or ischaemia-driven revascularisation at 30 days favoured PCI. However, secondary outcome events were less frequent in the CABG group at 5 years, showing a curve of events intersection between the groups compared (OR 1.39; 95% CI [1.13–1.71]; p=0.002).18
Finally, in the NOBLE trial, PCI (with a biolimus-eluting stent and 10% first-generation stents) was associated with worse clinical outcomes at 5 years compared with CABG. This difference was driven by higher rates of non-procedural MI and repeated revascularisation in the PCI group, with no significant differences in mortality. The mean SYNTAX score was 22.5 and 81% had distal LMCAD, of whom one-third were treated with a two-stent strategy. No benefit was observed with PCI in patients with a low SYNTAX score.19 In general terms, patients with a low to intermediate SYNTAX score, including those with isolated LMCAD, CABG and PCI, are associated with similar MACE rates.6 However, a less aggressive intervention with PCI could be the first choice, especially in elderly patients and those with comorbidities and high surgical risk.
In a meta-analyses pooling 4,394 patients from the four trials discussed above and judged by a heart team to be suitable candidates for either PCI or CABG, the revascularisation results showed no significant differences in mortality at 5 (pooling the four trials) or 10 years (including only patients from the SYNTAX and PRECOMBAT).24 The medium SYNTAX score was 25. However, significant differences in the risk of stroke, MI and need of revascularisation were detected. The stroke risk after 1 year of randomisation (HR 0.37; 95% CI [0.19–0.69]) and the procedural MI risk at 30 days (HR 0.67; 95% CI [0.48–0.93]; p=0.015) were lower in the PCI arm. Both the spontaneous MI rate (HR 2.35; 95% CI [1.71–3.23]; p<0.0001) and the need for a new revascularisation (HR 1.78; 95% CI [1.51–2.10]; p<0.0001) at the 5-years follow-up favoured CABG.
We can conclude the non-inferiority of PCI versus CABG with respect to survival, especially in patients with LMCAD with low to intermediate coronary anatomical complexity. Moreover, PCI is related to fewer periprocedural complications, such as stroke, MI, AF and bleeding. PCI is also related to a better and faster recovery of quality of life, a shorter sick leave, earlier angina relief and a lower 30-day MACE rate, contributing to a shorter psychological recovery and less depression.25 Thus, compared with CABG, PCI allows a more rapid recovery. In contrast, CABG is associated with fewer adverse events at 1 year and a more durable revascularisation with greater time free from angina and a lower rate of repeated revascularisation.25
Medical Treatment in Left Main Coronary Artery Disease
In addition to LM revascularisation, the use of pharmacological treatments, such as high-intensity statins, single antiplatelet therapy, DAPT, β-blockers, renin–angiotensin–aldosterone system inhibitors and anti-angina agents, is mandatory to improve outcomes.2
Although LM disease is heterogeneous, the current clinical guidelines recommend revascularisation (with the maximum level of evidence) for all patients with a ≥50% LM stenosis.1 Presenting with high-risk features, such as left ventricular dysfunction, heart failure, severe LM stenosis >70%, elevated left ventricular filling pressure, resting angina, resting ST-T electrocardiogram changes and prior MI, is associated with higher mortality compared with patients with preserved ejection fraction and less severe LM lesions (50–70%).26–28 Importantly, the strong recommendations in current clinical guidelines come from a subgroup analysis of RCTs conducted in the 1980s, when the current therapeutic arsenal and the more accurate evaluation of intermediate LM lesions with IVUS and pressure guidewire were not available. Furthermore, RCTs comparing revascularisation with optimal medical therapy in stable CAD had systematically excluded patients with LMCAD.29 For these reasons, the safety and suitability of optimal medical treatment in selected low-risk patients with LMCAD have not been well studied in the contemporary era. Considering not only CABG surgery, but also the highly complex LM-PCI risk, the costs and the hospital length of stay after these procedures, it is of special interest to properly identify low-risk patients who may benefit from deferred revascularisation, avoiding unnecessary surgical risk and costs, reducing complications and avoiding competitive flow after revascularisation in non-significant stenosis.
Although LM stenosis ≥70% is associated with excellent angiographic assessment with low interobserver variability, in those with intermediate stenosis (30–70%), the visual estimation is challenging.30 Revascularisation of angiographic LM intermediate lesions without haemodynamic or intravascular imaging assessment may result in unnecessary PCI or CABG.31
Optimisation of PCI
IVUS is the gold standard in the evaluation of intermediate LM lesions. IVUS improves the characterisation of the stenosis, allowing for a more accurate evaluation of lesion length, diameter and its location, distinguishing between shaft stenosis and stenoses compromising the bifurcation and identifying plaque burden and morphology (calcification versus thrombotic lesions). Thus, clinical guidelines recommend the use of IVUS in patients with LM intermediate stenosis to help define lesion severity.1 Moreover, the IVUS technique improves the PCI result by ensuring an adequate expansion and apposition of stents, which is of special interest in LM-PCI two-stent strategies because of its good prognostic value. Considering the potential risks and marked complications if the LM-PCI result is not adequate, it is highly recommended that intracoronary imaging is used during LM-PCI to achieve an optimal result.
Furthermore, IVUS use following LM-PCI was associated with a greater survival free of cardiac death, MI and target lesion revascularisation at 3 years, as well as a lower rate of probable and definite stent thrombosis in a propensity score-matched analysis including 1,670 patients undergoing DES implantation.32
Various studies have attempted to find a minimum lumen area (MLA) cut-off for LMCAD. In a study with 55 patients with ambiguous LMCAD, using IVUS and a pressure guidewire to calculate FFR, a strong correlation was demonstrated between FFR and minimum lumen diameter (MLD; r=0.79, p<0.0001), as well as MLA (r=0.74, p<0.0001).33 An MLD of 2.8 mm and an MLA of 5.9 mm2 strongly predicted the physiological significance of LM stenosis.33 However, in another study with 55 patients with isolated LM 30–80% stenosis, an IVUS-derived MLA <4.8 mm2 was considered a useful criterion for predicting FFR <0.80.34 In a prospective multicentre study with 354 patients with intermediate lesions in unprotected LMCAD evaluated with IVUS, deferring revascularisation in those with an MLA ≥6 mm2 was safe.35 Cardiac death-free survival was 97.7% in the deferred group, with no clinically significant difference compared with the revascularisation group. The correlation between IVUS and angiographic evaluation of significant stenosis was low. In that study, 43% of patients with angiographically significant LM stenosis had an MLA of ≥6 mm2 and more than 30% of patients with mild angiographic LM stenosis had an MLA <6 mm2.35
With regard to the debate regarding MLA cut-off values (6 versus 4.5 mm2) there are several aspects to consider. First, the MLA cut-off may differ between populations, probably due to variances in the size of the coronary arteries. For example, the study using an MLA cut-off of 4.8 mm2 for an FFR of <0.80 was validated in a Korean population, whereas the study in which the MLA cut-off was 6 mm2 was performed in a Western population.34,35 A consensus recommendation on the role of imaging to assess lesion significance suggested that an LM IVUS-derived MLA >6 mm2 can be considered non-ischaemic, whereas an MLA ≤4.5 mm2 can be considered ischaemia-generating.36 When LM IVUS-derived MLA is between 4.5 mm2 and 6 mm2, additional ischaemia assessment is recommended. Second, due to the life-threatening risk of not revascularising a significant LM stenosis, the MLA cut-off should have a very high sensitivity (close to 100%) at the expense of a certain lower degree of specificity. The value of 6 mm2 meets these criteria, whereas the MLA cut-off of 4.8 mm2 only reaches 89% sensitivity. Furthermore, the value of 6 mm2 has been validated by a large prospective study, namely the LITRO clinical trial.37 Thus, a value of 6 mm2, more than a predictive cut-off of ischaemia derived from a correlation with FFR, should be used as the cut-off for the safe deferral of revascularisation.37 Finally, the role of IVUS in the anatomical setting of the LM is supported by the excellent correlation of MLA with FFR, which is not present in the rest of the coronary segments. Therefore, MLA measurement of non-LMCA lesions is not recommended for the assessment of lesion significance due to variations according to vessel calibre and subtended myocardium.36
IVUS and FFR evaluate coronary stenosis with an anatomical and a haemodynamic approach, respectively, and are complementary techniques that may improve PCI outcomes. When IVUS assessment shows an MLA between 4.5 and 6 mm2, FFR is helpful in determining the severity of the stenosis. However, the haemodynamic assessment may be less accurate in patients with lesions distal to the target stenosis.38
In a prospective multicentre registry with 300 patients with intermediate LM stenosis, a deferred revascularisation guided by the instantaneous wave-free ratio (iFR) was feasible, but with moderate concordance with FFR (80%).39 In cases of disagreement, IVUS was useful to indicate revascularisation.39
In summary, the use of both intracoronary imaging and functional assessment helps interventional cardiologists with the complex decision-making process of the LM-PCI. Optical coherence tomography (OCT) can provide high-resolution images and has been shown to correlate well with IVUS measurements. However, OCT requires blood clearance, which is a strong limitation for imaging ostial LM.1 A proposed algorithm for the management of LM disease is shown in Figure 2.
PCI Technical Considerations
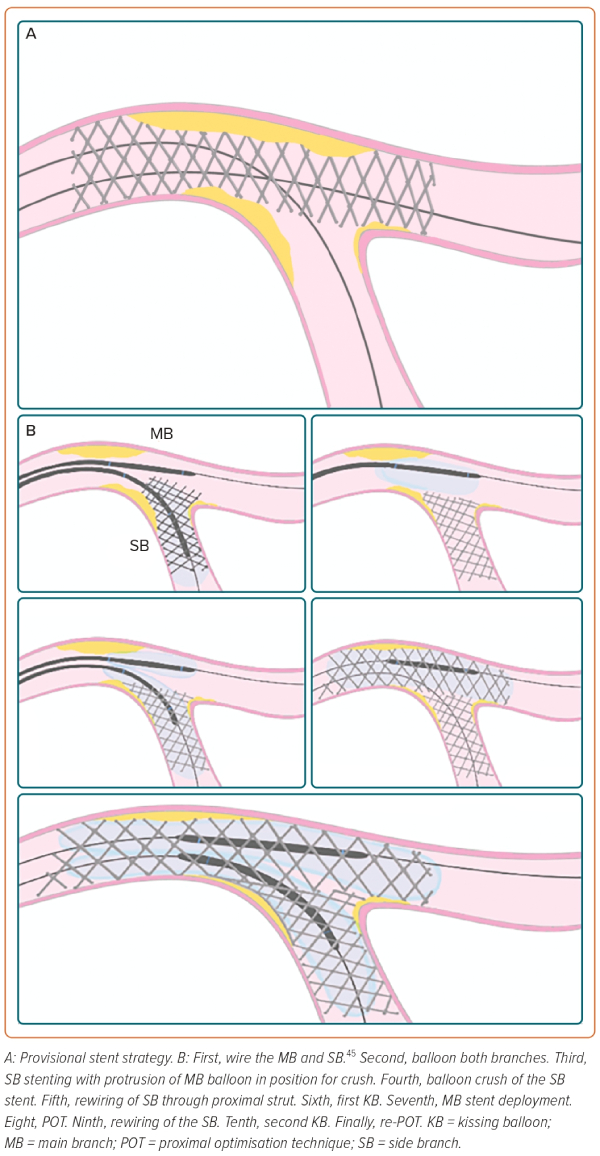
A high proportion of patients with unprotected LMCAD present with lesions involving the distal LM bifurcation.28 This situation is associated with worse PCI outcomes compared with PCI of ostial and shaft LM stenosis. A bifurcation lesion is a ‘coronary artery narrowing occurring adjacent to, and/or involving, the origin of a significant side branch (SB), which is a branch that the operator does not want to lose.40 According to the 15th consensus document from the European Bifurcation Club, some technical considerations should be kept in mind for bifurcation stenting, especially in LM, as outlined below.40
Operators should adhere to the KISS (keep it simple and safe) principle.
Systematically wire both branches. Concerns regarding SB occlusion and expected difficulties in rewiring are key factors in the stenting strategy decision-making process.
Attempt to use the fewest number of stents with a provisional stent (PS) strategy in the majority of cases. Two-stent techniques are a potential risk factor for stent thrombosis and should be reserved for highly complex LM-PCI involving the SB. When using a two-stent strategy, the kissing balloon technique is mandatory to facilitate access to the SB. In the EXCEL and NOBLE trials, the PS strategy was performed in the vast majority of patients included.18,19 PS has been associated with better outcomes compared with a planned two-stent approach in RCTs of non-LM bifurcation lesions.41 Nevertheless, in the DKCRUSH trial (n=482), the double kissing (DK) crush approach resulted in a lower target lesion failure and stent thrombosis rate at the 3-year follow-up compared with PS in patients with LM bifurcation lesions.42 Furthermore, in a systematic review and network meta-analysis with 5,711 patients, DK crush, compared with other bifurcation PCI techniques, such as crush, culotte and T stenting/T and protrusion, was associated with fewer MACE (driven by lower rates of repeat revascularisation) with no significant differences in cardiac death, MI or stent thrombosis.43 DK crush was also superior to PS when SB lesion length was ≥10 mm.43 The EBC MAIN trial compared PS with a two-stent strategy in 467 patients with bifurcation distal LMCAD.44
As explained previously, the DKCRUSH-V trial addressed a similar question, showing better outcomes in the double stenting group, especially in those treated with the DK crush technique.40 However, important methodological differences between these two trials should be noted. First, all patients included in the EBC MAIN trial had significant ostia stenosis of both the left anterior descendent artery and the circumflex artery, but patients included in the DKCRUSH-V trial had a higher SYNTAX score (31 versus 23) and greater SB lesion length (16 mm versus 7 mm). Second, the EBC MAIN trial, in contrast with the DKCRUS-V trial, did not compare a one- versus two-stent strategy, but PS (which could entail extension to two stents) with an upfront assignment to use the two-stent technique. Indeed, 45% and 22% of patients in the PS arm had two stents implanted in the DKCRUSH-V and EBC MAIN trials, respectively. In the EBC MAIN trial, the majority of two-stent procedures were culotte (53%) or T stenting/T and protrusion (33%). Outcomes definitions also differed between the two studies.
In DKCRUSH-V, the primary endpoint was target lesion failure and stent thrombosis was the safety endpoint. In the EBC MAIN trial, no significant differences were found in the primary composite endpoint of 1-year death, MI and target lesion revascularisation (14.7% in the PS arm versus 17.7% in the upfront two-stent group; HR 0.8; 95% CI [0.5–1.3]). Moreover, no significant difference was detected for any of the individual components of the primary endpoint. The rates of stent thrombosis were similar (1.7% in the PS group and 1.3% in patients treated with two stents upfront). Finally, angiographic follow-up was undertaken in the DKCRUSH-V trial, demonstrating a sudden spike in target lesion failure at 12 months (from 2.9% at the 30-day follow-up to 10.7% at the 1-year follow-up; Figure 3).
Use a proximal optimisation technique to tailor and reach an optimal match with the patient’s anatomy to pursue well-apposed and well-expanded stents, avoiding overlapping.40
The use of intracoronary imaging during LM-PCI is highly recommended.
Conclusion
LM revascularisation is still a clinically challenging decision. Although CABG is considered standard therapy, PCI is a good alternative with similar survival results, particularly in patients with low or intermediate coronary anatomy complexity. The heterogeneity in the methods of the RCTs comparing both revascularisation techniques may explain the discordant results. PCI and CABG should no longer be considered as competitive, but rather as complementary strategies. The coronary anatomy and clinical characteristics, the centre and operator expertise and patient preferences are key factors to be judged by the heart team. Assessment of LM stenosis with IVUS and FFR is highly recommended because these strategies improve the identification of significant stenosis, deferring unnecessary revascularisation and improving outcomes when PCI is performed.
Despite decades of continuous research into the optimum strategy to treat LMCAD, important gaps in evidence remain. Although there are some observational data, it has not determined whether the current arsenal of pharmacological therapies could be a safe strategy in patients presenting with intermediate LM stenosis without high-risk factors. In this direction, IVUS and FFR may help identify those asymptomatic patients with low-risk LM stenosis that can benefit from deferral of unnecessary CABG or high-risk PCI with a very low rate of events during follow-up under medical treatment. Moreover, IVUS is highly recommended in LM-PCI to improve outcomes. Finally, the different PCI strategies combining the one-stent versus the two-stent approach may improve LM-PCI results, which may be a target for future clinical research.