For more than 50 years, oral vitamin K antagonists (VKAs) were the choice of anticoagulant for the long-term treatment and prevention of arterial and venous thromboembolic events (VTE). VKA treatment is safe and effective, if a high time in therapeutic range is achieved. However, achieving a stable, therapeutic international normalised ratio can prove challenging in the context of drug and food interactions and liver disease, resulting in either an increased risk of thromboembolism due to undertreatment or bleeding due to overtreatment. In recent years, four direct oral anticoagulants (DOACs), dabigatran, rivaroxaban, apixaban and edoxaban, have been compared with warfarin for stroke prevention in AF, in large, phase 3, randomised, controlled trials (RCTs).
Various terms have been used to describe these drugs, including new/ novel oral anticoagulants or non-vitamin K oral anticoagulants. The International Society on Thrombosis and Haemostasis recommends using the term ‘DOAC’.1 These anticoagulants directly inhibit specific proteins within the coagulation cascade; in contrast, VKAs inhibit the synthesis of vitamin K-dependent clotting factors.
Dabigatran, a direct thrombin inhibitor, and rivaroxaban, apixaban and edoxaban, the factor Xa inhibitors, produce a more predictable, less labile anticoagulant effect; they have been shown to be at least as safe and effective as warfarin in stroke prevention in AF. As the availability of DOACs has expanded anticoagulant treatment options, it is important that clinicians familiarise themselves with these novel agents. They are licensed for use in stroke prevention in non-valvular AF, the treatment of VTE and as thromboprophylaxis following major orthopaedic surgery. Rivaroxaban has also been approved in Europe for the prevention of atherothrombotic events following acute coronary syndrome (ACS). The purpose of the present article was to summarise the available trial data and provide useful clinical guidance on the different DOACs for stroke prevention in non-valvular AF.
Efficacy and Safety
Non-Valvular AF
DOACs have been studied extensively, and results have shown that they are at least as effective and safe as treatment with warfarin in stroke prevention in non-valvular AF. DOACs are not licensed for use in patients with mechanical heart valves. The Randomised, Phase II Study to Evaluate the Safety and Pharmacokinetics of Oral Dabigatran Etexilate in Patients After Heart Valve Replacement (RE-ALIGN) was terminated early due to increased rates of thromboembolic and bleeding complications in patients with mechanical heart valves taking dabigatran compared with warfarin patients.2
In the phase 3 clinical trials, many patient subgroups were excluded or underrepresented. In contrast to the RCTs, patients in the real world demonstrate heterogeneity with a greater variation in stroke risk, renal impairment, liver disease and other conditions associated with an increased risk of bleeding. Real-world data are therefore needed to assess whether the efficacy and safety of DOACs achieved in patients with non-valvular AF in RCTs translate into routine clinical practice.
In the Randomized Evaluation of Long Term Anticoagulant Therapy With Dabigatran (RE-LY) study, both doses of dabigatran (110 mg twice daily and 150 mg twice daily) were shown to be non-inferior to warfarin in preventing stroke and systemic embolism (p<0.001 in both arms), with the higher dose also being superior to warfarin (p<0.001).3 Interestingly, the rates of haemorrhagic stroke were significantly lower in both dabigatran arms (p<0.001), as well as rates of life-threatening bleeding (low dose: p<0.001, high dose: p=0.04) and intracranial bleeding (ICH) (p<0.001 in both arms). However, the rate of gastrointestinal (GI) bleeding was significantly higher in the higher-dose dabigatran arm (p<0.001). The results of a systematic review and meta-analysis of 348,750 patients on dabigatran in real-world clinical practice were similar, with higher GI bleeding rates compared to warfarin (p=0.041), but a lower rate of ICH (p<0.001).4 In the RE-LY study, there was a non-significant increase in myocardial infarction (MI) with dabigatran compared with warfarin. This has not been reproduced in the real-world data, with a US Food and Drug Administration study of 134,000 Medicare patients5 and a multicentre registry of 555 patients6 showing no difference in the rates of MI.
In an efficacy and safety study of Rivaroxaban With Warfarin for the Prevention of Stroke and Non-Central Nervous System Systemic Embolism in Patients With Non-Valvular Atrial Fibrillation (ROCKET-AF) trial, rivaroxaban was shown to be non-inferior to warfarin in preventing stroke and systemic embolism (p<0.001);7 the rates of ICH and fatal bleeding were significantly lower in those treated with rivaroxaban (p=0.02 and p=0.003, respectively). However, there was a significant increase in major GI bleeding in the rivaroxaban arm (p<0.001), although there was no difference in all-cause mortality. In the real-world observational Xarelto for Prevention of Stroke in Patients With Atrial Fibrillation (XANTUS) study, both lower thrombotic and bleeding rates were reported compared with the ROCKET-AF trial, including a lower rate of GI bleeding (0.9 versus two events/100 patient years, respectively).8 In the XANTUS study, patients had a mean CHADS2 score of 2 compared to 3.5 in the ROCKET-AF study, which could explain the discrepancy in the rates of thrombotic complications.
Apixaban was shown to be non-inferior (p<0.001) and superior to warfarin (p<0.01) for the prevention stroke and systemic embolism in the Apixaban for Reduction in Stroke and other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial. Rates of haemorrhagic stroke and ICH were also significantly lower (p<0.001 for both).9 There was no significant difference in ischaemic stroke, but all-cause mortality was significantly lower (p<0.001). These results were corroborated by a retrospective analysis, which showed a significant reduction in the risk of stroke and systemic embolism (p=0.04) and a reduced risk of major bleeding, including GI bleeding and ICH (p<0.001).10
Both doses of edoxaban (30 mg once daily and 60 mg once daily) were shown to be non-inferior to warfarin (p=0.005 and p<0.001) and superior in the case of the higher dose (p=0.02) in preventing stroke and systemic embolism in the Effective Anticoagulation with Factor XA Next Generation in Atrial Fibrillation – Thrombolysis In Myocardial Infarction 48 (ENGAGE AF-TIMI) 48 trial.11 However, edoxaban at the lower dose of 30 mg has been shown to be inferior to warfarin in preventing ischaemic stroke (p<0.001). There was an increase in the rate of stroke in edoxaban patients with a creatinine clearance of >95 ml/min, although this was not statistically significant (p=0.08).12 Both doses were associated with lower rates of bleeding, including ICH and major and life-threatening bleeding (p<0.001 for all). The only exception was the higher dose, which was associated with a higher rate of GI bleeding (p=0.03).
A meta-analysis of the four phase 3 clinical trials was performed to assess the safety and efficacy of DOACs in patients with AF and heart failure (HF).13 A total of 26,384 (48 %) of the 55,011 patients enrolled in the four trials had a diagnosis of HF (defined according to individual trial definitions). There were similar rates of stroke and systemic embolism (p=0.68) and major bleeding (p=0.21) in HF and no-HF patients. Those with HF had reduced rates of total bleeding (p<0.01) and ICH (p<0.01), but a higher rate of all-cause death (p<0.01) and cardiovascular death (p<0.01) compared to no-HF patients treated with DOACs (this finding did not appear to be affected by the choice of oral anticoagulant). The rates of stroke and systemic embolism, major bleeding and ICH were significantly reduced in those treated with DOACs compared with warfarin, regardless of the presence or absence of HF (p>0.05 for each interaction). These findings suggest that DOACs are safe and efficacious in patients with AF, regardless of whether they have HF or not.
Acute Coronary Syndrome
At present, rivaroxaban is the only DOAC licensed for use in ACS. Data from the Anti-Xa Therapy to Lower Cardiovascular Events in Addition to Standard Therapy in Subjects With Acute Coronary Syndrome ACS 2–Thrombolysis In Myocardial Infarction 51 (ATLAS ACS 2-TIMI 51) trial showed that rivaroxaban at either 2.5 mg twice daily or 5 mg twice daily, in addition to either aspirin or dual antiplatelet therapy (DAPT), significantly reduced the rate of death from cardiovascular causes, compared to the placebo (p=0.008).14 Although there was a higher rate of major bleeding with rivaroxaban (p<0.001), there was no statistically-significant difference in fatal bleeding between rivaroxaban and placebo. Subgroup analysis revealed that major or minor bleeding and fatal bleeding rates were lower in the rivaroxaban 2.5 mg twice daily arm compared to the 5 mg twice daily arm (p=0.021 and p=0.044), but with no significant difference in death from cardiovascular causes. An additional subgroup analysis, evaluating patients who had undergone percutaenous coronary intervention (PCI) as part of the index event or previous stent insertion, demonstrated a reduction in stent thrombosis (p=0.023) and mortality (p=0.039) with the use of rivaroxaban 2.5 mg twice daily, in addition to DAPT, compared to placebo and DAPT.
In the Open-label, Randomized, Controlled, Multicenter Study Exploring Two Treatment Strategies of Rivaroxaban and a Dose-Adjusted Oral Vitamin K Antagonist Treatment Strategy in Subjects With AF Who Undergo PCI (PIONEER AF-PCI) trial, patients with non-valvular AF undergoing PCI and stent placement were randomised to receive either low-dose rivaroxaban (15 mg once daily) plus a P2Y12 inhibitor for 12 months, very low-dose rivaroxaban (2.5 mg twice daily) plus DAPT for 1, 6 or 12 months or standard therapy with a VKA and DAPT for 1, 6 or 12 months.15 Death rates from cardiovascular causes, MI and stroke were similar in the three groups (although the similar efficacies should be interpreted with caution, due to broad confidence intervals), but clinically-significant bleeding rates were lower in both rivaroxaban groups (p<0.001 for both doses). These data suggest that rivaroxaban, in addition to either a P2Y12 inhibitor or DAPT, is safer in terms of bleeding risk in patients with AF undergoing PCI than warfarin. The other DOACs are currently being evaluated for their role in PCI. The results of the Evaluation of Dual Therapy With Dabigatran vs. Triple Therapy With Warfarin in Patients With AF That Undergo a PCI With Stenting (RE-DUAL-PCI),16 Apixaban Versus Warfarin in Patients with AF and acute coronary syndrome or PCI (AUGUSTUS) (apixaban)17 and Edoxaban Treatment Versus Vitamin K Antagonist in Patients With AF an Undergoing PCI (ENTRUST AF-PCI; edoxaban) trials18 are awaited.
Selecting a Direct Oral Anticoagulant
In comparison to conventional treatment with VKAs, DOACs offer many treatment advantages, including fixed dosing, fewer drug and dietary interactions, rapid onset and short half-lives, which can preclude the need for peri-procedural bridging, as well as no monitoring requirement. It is also important to be aware of potential disadvantages: there is no specific reversal agent (with the exception of idarucizumab for dabigatran), there is a need for avoidance or dose reduction in renal impairment and the short half-lives necessitate strict adherence. It is therefore important to take into account patient-specific factors in order to tailor anticoagulant treatment to the individual.
High CHA2DS2-VASc Score
For patients with a high risk of thromboembolic disease and acceptable bleeding risk, consider using dabigatran 150 mg twice daily, as this is the only DOAC with superior efficacy in the reduction of ischaemic stroke, compared to warfarin.3
Bleeding Risk
Most of the available data evaluating DOACs and bleeding risk have been taken from AF trials. In patients with a high risk of GI bleeding, consider avoiding 150 mg dabigatran, rivaroxaban and 60 mg edoxaban, as these are associated with higher GI bleeding rates compared to warfarin.3,7,11 Rates of GI bleeding associated with 110 mg dabigatran and apixaban were similar to those in the warfarin arms of their trials. Dabigatran should also be avoided in patients with dyspepsia or peptic ulcer disease, as symptoms can worsen. All DOACs are associated with a lower risk of ICH compared with warfarin.3,7,9,11
Renal Impairment
Creatinine clearance should be calculated using the Cockcroft– Gault equation prior to commencing a DOAC. Renal clearance of dabigatran accounts for 85 % of total clearance, and it is therefore contraindicated in those with a creatinine clearance of <30 ml/min. A study by Chan et al. showed that the risk of hospitalisation or death from bleeding was higher in dialysis patients taking dabigatran and rivaroxaban compared with warfarin (p=0.0001 and p=0.04).19 Dose reductions of rivaroxaban, apixaban and edoxaban could be required, depending on the creatinine clearance, but these drugs are not contraindicated unless creatinine clearance is <15 ml/min. A systematic review and meta-analysis of nine RCTs including 54,667 patients with renal impairment showed a significantly reduced risk of major bleeding in those taking DOACs, with a creatinine clearance of 50–80 ml/min (risk ratio: 0.87 [95 % confidence interval: 0.81–0.93]), and a non-significant decrease in those with a creatinine clearance of <50 ml/min (risk ratio: 0.83 [95 % confidence interval: 0.68–1.02]), compared to warfarin.20 Apixaban was associated with a decreased rate of major bleeding compared with other DOACs in patients with a creatinine clearance of <50 ml/min. A retrospective matchedcohort study assessed the safety and efficacy of apixaban versus warfarin in patients with a creatinine clearance of <25 ml/min, serum creatinine concentration of >2.5 mg/dl or on dialysis.21 There was a non-significant decrease in major bleeding in those who received apixaban compared with warfarin (9.6 % versus 17.8 %, p=0.149), and a similar occurrence of stroke between the two groups (7.5 % in both). Further studies are needed to establish whether apixaban is safe and effective in those with a creatinine clearance of <15 ml/min.
Adherence
Obamiro et al. assessed patients’ anticoagulation knowledge, and showed that patients taking DOACs were less likely to view missing a dose as a problem, compared to those taking warfarin (p<0.05).22 Unlike warfarin, DOACs have a rapid onset and offset of anticoagulant activity, and therefore, 1 day without the drug will render the patient unanticoagulated. Patients should therefore be counselled about the risk of missing a dose of a DOAC. If there are concerns regarding non-adherence, warfarin, due to its long half-life, should be considered.
Pregnancy
Dabigatran has been shown to cross the placenta.23 Animal studies have demonstrated pregnancy loss and foetal harm with both dabigatran and rivaroxaban.24,25 It is unknown whether they are secreted in breast milk. Data regarding edoxaban and apixaban are limited. DOACs should therefore be avoided in pregnancy and breastfeeding.
Cancer
In patients with cancer-associated VTE, low molecular weight heparin (LMWH) is currently the treatment of choice, due to its superiority in preventing recurrent thrombosis and a reduction in bleeding, compared to warfarin.26–29 DOACs are being evaluated for their role in cancer-associated VTE in a number of ongoing trials, including Anticoagulation Therapy in Selected Cancer Patients at Risk of Recurrence of Venous Thromboembolism (SELECT-D; rivaroxaban),30 Apixaban for the Treatment of Venous Thromboembolism in Patients With Cancer (CARAVAGGIO trial; apixaban),31 Hokusai VTE–cancer (edoxaban)32 and DOACs versus LMWH ± Warfarin for VTE in Cancer: A Randomized Effectiveness Trial (CANVAS; all four DOACs).33
The management of AF in patients with cancer can be challenging due to a number of factors, including increased bleeding risk, drug interactions and frequent invasive procedures. Not only is there a lack of evidence regarding the use of LMWH in AF but it is also not licensed for this indication. In patients taking chemotherapy, dabigatran might be preferable to other DOACs due to fewer drug interactions in view of its non-CYP-mediated metabolism.34
Patients with cancer were excluded from the AF trials, and so there are limited data regarding the use of DOACs in cancer patients with AF. Therefore, further studies are needed to establish their role in this situation. However, in the VTE trials, preliminary evidence suggests that DOACs were safe and efficacious in cancer patients who subsequently developed AF. Furthermore, an observational study on cancer patients with AF showed that bleeding rates were similar or lower in those taking DOACs compared to warfarin.35 Although there was no difference in the risk of ischaemic stroke, DOACs were superior to warfarin in lowering the risk of incident VTE.
Drug Interactions
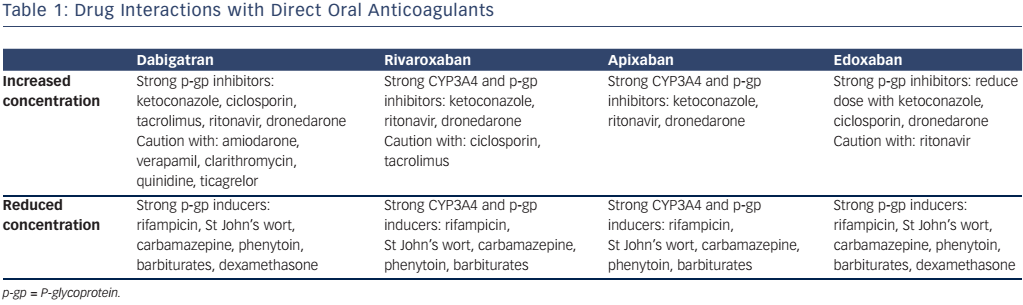
Although drug interactions are less frequent than with warfarin, it is important to be aware of which drugs interfere with DOAC metabolism. The hepatic enzyme CYP3A4 is important in the metabolism of rivaroxaban and apixaban, and all DOACs are substrates of the P-glycoprotein transporter system. Enzyme inducers can cause a reduction in DOAC plasma concentration, and could therefore increase the risk of thromboembolic events, while inhibitors potentiate the DOAC concentration, which could result in bleeding. The important drug interactions are highlighted in Table 1.
Measurement of Direct Oral Anticoagulant Activity
Because DOACs have a predictable anticoagulant effect and are administered at a fixed dose, they do not require routine monitoring.
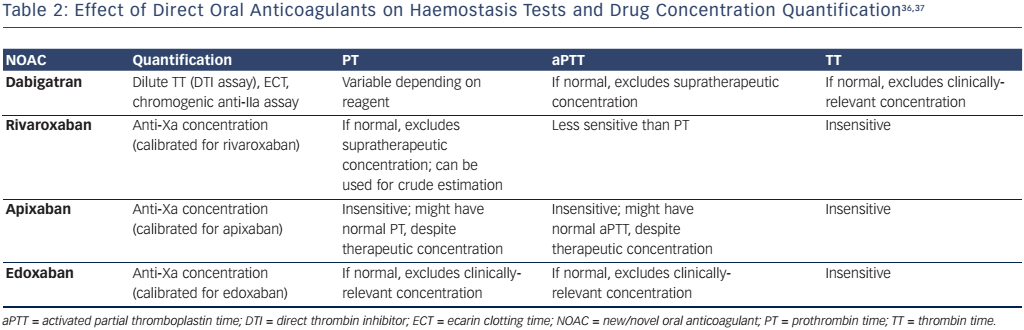
However, there could be circumstances in which the measurement of DOAC activity would be useful, for example, in bleeding patients or those requiring emergency surgery, patients with recurrent thrombosis on treatment or if there are concerns regarding adherence. Table 2 outlines how each DOAC affects conventional tests of haemostasis, and which tests should be performed to quantify drug concentration. To enable interpretation of the coagulation screen, each laboratory should be aware of the sensitivity of their reagents to the different DOACs
Perioperative Management
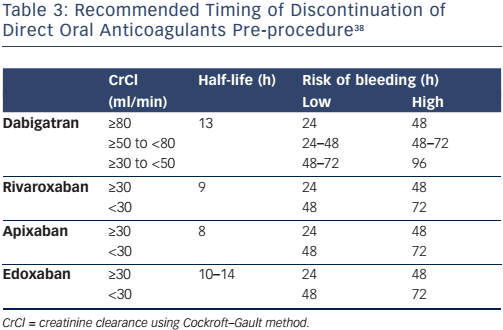
The timing of stopping a DOAC prior to an invasive procedure or operation depends on the half-life of the drug, creatinine clearance and the risk of bleeding (Table 3). Invasive procedures associated with a very low risk of bleeding, such as dentistry, joint injections, cataract surgery and pacemaker insertion, might not require interruption of anticoagulation, whereas high-risk procedures, such as spinal anaesthesia, coronary artery bypass, heart valve surgery and neurosurgery, will require discontinuation of the DOAC at least 48 hours before. Anticoagulation can be recommenced at 6–12 hours following low-risk procedures if haemostasis has been achieved, and at 48 hours following high-risk procedures.
Management of Bleeding
With the exception of dabigatran, there is currently no licensed reversal agent for DOACs. Andexanet alfa has been shown to rapidly reverse the antifactor Xa activity in those with acute major bleeding associated with rivaroxaban or apixaban,39 but is not yet available in clinical practice. It is a recombinant modified human factor Xa decoy protein that is catalytically inactive due to the substitution of the active-site serine with alanine. It lacks the gamma-carboxyglutamic acid domain, preventing it from binding to phospholipid membranes, but retains the ability to bind to factor Xa inhibitors with a high affinity. Ciraparantag (aripazine) is a broad-spectrum reversal agent that binds to the oral factor Xa and direct thombin inhibitors, unfractionated heparin and LMWH, and is currently being evaluated in phase 2 clinical trials. If the patient is taking an oral factor Xa inhibitor and is unresponsive to general haemostatic measures, prothrombin complex concentrate (PCC) at a dose of 25–50 units/kg should be considered. Idarucizumab, a monoclonal antibody, binds free and thrombin-bound dabigatran, thereby preventing it from exerting its anticoagulant effect, and is now licensed for use. The recommended dose is 5 g, given intravenously when rapid reversal is required in those who need emergency surgery or those with uncontrolled bleeding. A second dose might be considered if bleeding recurs or if there is a potential for life-threatening rebleeding in association with prolonged clotting times.
Local protocols should be developed to ensure that patients on DOACs with serious or life-threatening bleeding are managed appropriately. The general approach to a bleeding patient on any form of anticoagulation should be as follows:40
- stop the anticoagulant;
- request an urgent coagulation screen (activated partial thromboplastin time, prothrombin time, thrombin time [TT]), full blood count and group and save;
- haemodynamic monitoring and resuscitation with fluid and blood products, if indicated;
- mechanical compression or surgical or radiological intervention to identify and treat the source of bleeding.
If the patient is taking a DOAC:
- establish the timing of the last dose; if ingestion was within the past 2 hours, consider oral activated charcoal;
- renal function should be assessed urgently; this allows estimation of the remaining duration of drug exposure;
- if the patient is taking dabigatran, a normal TT excludes a clinically-relevant dabigatran concentration; if the TT is prolonged, a dabigatran effect might be present;
- a normal coagulation screen excludes supratherapeutic concentrations of rivaroxaban and edoxaban, but might not exclude clinically-relevant drug concentrations;
- the coagulation screen might be normal in patients taking apixaban;
- consider intravenous tranexamic acid and seek advice from a haematologist regarding the administration of PCC, or idarucizumab if the patient has taken dabigatran.
Conclusion
Large, phase 3 clinical trials have shown that DOACs are at least as safe and effective as VKAs in the prevention of stroke in non-valvular AF. Real-world data have allowed us to determine the efficacy and safety of these drugs in everyday clinical practice, outside the confines of RCTs, and thus far, have shown similar outcomes, with reduced rates of major bleeding, including ICH and increased or similar rates of GI bleeding.
Betrixaban, an oral factor Xa inhibitor, has shown promise in extended VTE prophylaxis41 and stroke prevention42 in those hospitalised with an acute medical illness. Due to its distinct pharmacokinetic profile with minimal renal clearance and a long half-life, this might overcome some of the limitations of the other DOACs in clinical practice. The availability of andexanet alfa and ciraparantag will address the unmet need for reversal agents in the management of DOAC-associated major and life-threatening bleeding.
DOACs have many advantages over VKAs, including a rapid onset and offset of anticoagulant effect, fixed dosing, fewer drug and dietary interactions and no monitoring requirement; these make attractive options for anticoagulation. As there are several agents available, it is important that clinicians are familiar with the efficacy and safety profiles of DOACs, based on both the trial and real-world data, to counsel and tailor treatment to the individual patient.