In recent years, coronary functional disorders (CFD), such as epicardial or microvascular coronary spasm and coronary microvascular dysfunction (CMD), have gained increased attention in scientific and clinical settings as mechanisms of angina with non-obstructed coronary arteries (ANOCA).
The pathophysiology of CFD includes structural mechanisms (capillary rarefaction, arterial wall thickening and perivascular fibrosis), functional mechanisms (endothelial and/or vascular smooth muscle cell dysfunction), and myocardial mechanisms (left ventricular hypertrophy, myocardial infiltration and increased diastolic pressure).1 CFDs have significant implications for patient outcomes as they increase the risk of recurrent angina, reduce quality of life, and are associated with future adverse cardiovascular events.2–4 Therefore, effective diagnosis and management of CFD are crucial for improving patient outcomes. Studies within the past two decades have shown a high prevalence of CFD in ANOCA patients (53–86%).5–10 However, many of these studies did not include comprehensive coronary functional testing (CFT), which should involve coronary spasm provocation testing and the assessment of CMD.11
The diagnosis of CMD can be established invasively by measuring a diminished coronary flow reserve (CFR) and/or an increased hyperaemic microvascular resistance (HMR).12 Currently, different invasive measurement techniques, such as Doppler or thermodilution techniques, are used to assess CMD. Doppler flow-derived measurements are generally considered more reliable compared to the bolus-thermodilution technique.13
Recently, Konst et al. reported the prevalence of CFD among ANOCA patients using spasm provocation testing and the assessment of microvascular function with the thermodilution technique.10 In this study, we investigated the prevalence of CFD using acetylcholine (ACh) spasm provocation testing and intracoronary Doppler-based evaluation of coronary microvascular function.
Methods
Study Design
In this single-centre study, we enrolled consecutive stable patients with ANOCA (mostly from our ANOCA outpatient clinic) who underwent a scheduled comprehensive assessment of coronary reactivity, including ACh spasm testing and assessment of coronary microvascular function. All the patients had angina or an angina equivalent, such as dyspnoea or unobstructed coronary artery disease, defined as <50% stenosis and/or fractional flow reserve (FFR) >0.80. The study protocol adhered to the principles of the Declaration of Helsinki and received approval from the local ethics committee (Ethik-Kommission Tübingen, 278/2018/BO1). Assessment of coronary tortuosity was part of the structured analysis.
Coronary Spasm Provocation Testing
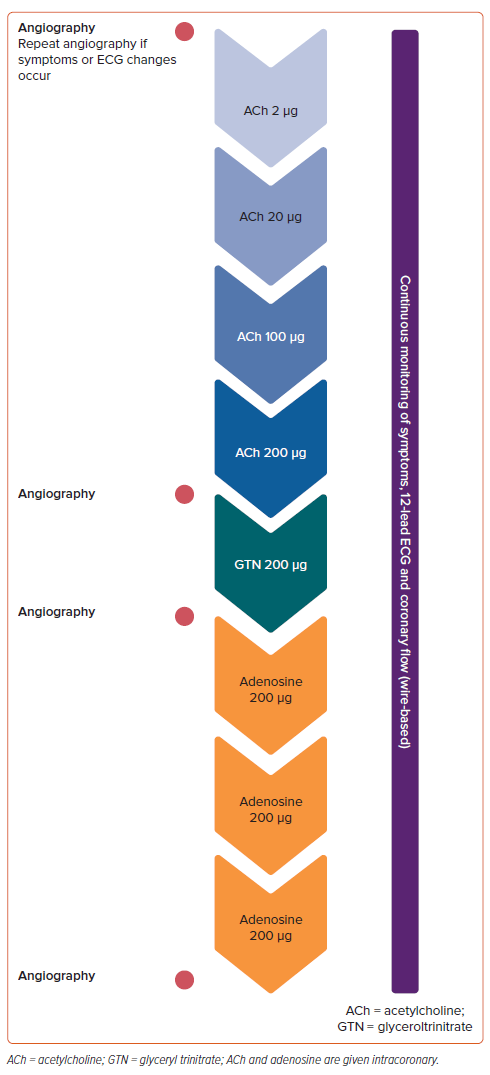
All patients underwent invasive coronary angiography, and severe stenoses (>90%) were ruled out by angiographic assessment before undergoing ACh provocation testing, as previously described (Figure 1).14 Haemodynamic relevance of intermediate coronary stenoses was assessed by FFR measurements during microvascular function testing following ACh testing. Briefly, during ACh testing, incremental doses of ACh (2, 20, 100 and 200 µg) were manually injected into the left coronary artery (LCA) via the guiding catheter. During and after each dose, we assessed the patient’s symptoms, 12-lead ECG, and epicardial vasoconstriction on angiography. New onset ischaemic ECG changes were defined as ST-segment depression or elevation ≥0.1 mV or transient T wave inversion in at least two contiguous leads. The presence of ≥90% focal or diffuse coronary diameter reduction compared to the relaxed state after intracoronary glyceryl trinitrate indicated epicardial spasm, while microvascular spasm was defined by the reproduction of symptoms and ischaemic EGC shifts without epicardial spasm, according to the COVADIS criteria.15,16 In cases where the LCA exhibited no signs of coronary spasm, we also conducted ACh testing on the right coronary artery (RCA) using 80 µg ACh.
Assessment of Coronary Microvascular Function
After spasm provocation testing, we performed the guidewire-based assessment of coronary microvascular function based on CFR and HMR measurements.14 In brief, after normalisation, a Philips Volcano ComboWire XT guidewire, equipped with a distal pressure sensor as well as a Doppler flow probe, was advanced to the proximal-to-mid-portion of the left anterior descending artery (LAD). Maximum hyperaemia was induced through three intracoronary injections of 200 µg adenosine, ensuring the reliability of CFR, FFR and HMR assessments.17 Diminished CFR was defined as CFR <2.5 based on previous data, while increased HMR was defined as HMR >2.5.16,18–20
Assessment of Coronary Tortuosity
The LCA and RCA were subjected to visual examination using analysis tools integrated within the WebPAX electronic database (Heart Imaging Technologies). The angiographic assessments were conducted through multiple projections and cross-referenced in diverse perspectives to mitigate potential projection errors. The determination of coronary tortuosity severity, as per the criteria established by Eleid et al., was based on the count and angles of coronary artery curves during late diastole.21 The classification of tortuosity severity was as follows: mild – three consecutive curves with angles between 45 and 90°, moderate – three or more curves with angles between 90 and 180°, severe – two curves within a segment with angles of 180°, and non-tortuous – curves with angles <45°.
Statistical Analysis
Data are presented as median (Q1–Q3) or absolute numbers (percentage) as appropriate. Data analysis was conducted using GraphPad Prism 5.01 (GraphPad) and SPSS 23.0 (IBM). We used the Mann-Whitney test for continuous variables and Fisher’s exact test to compare categorical variables. A two-tailed p-value <0.05 was considered statistically significant.
Results
Study Population
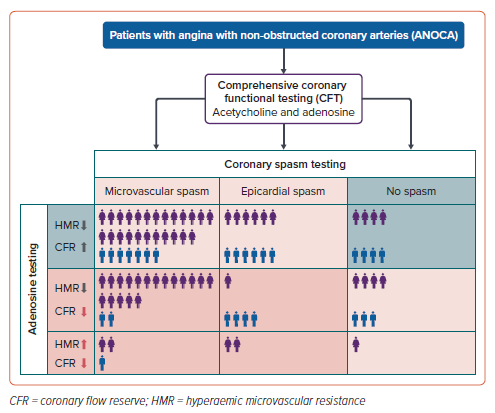
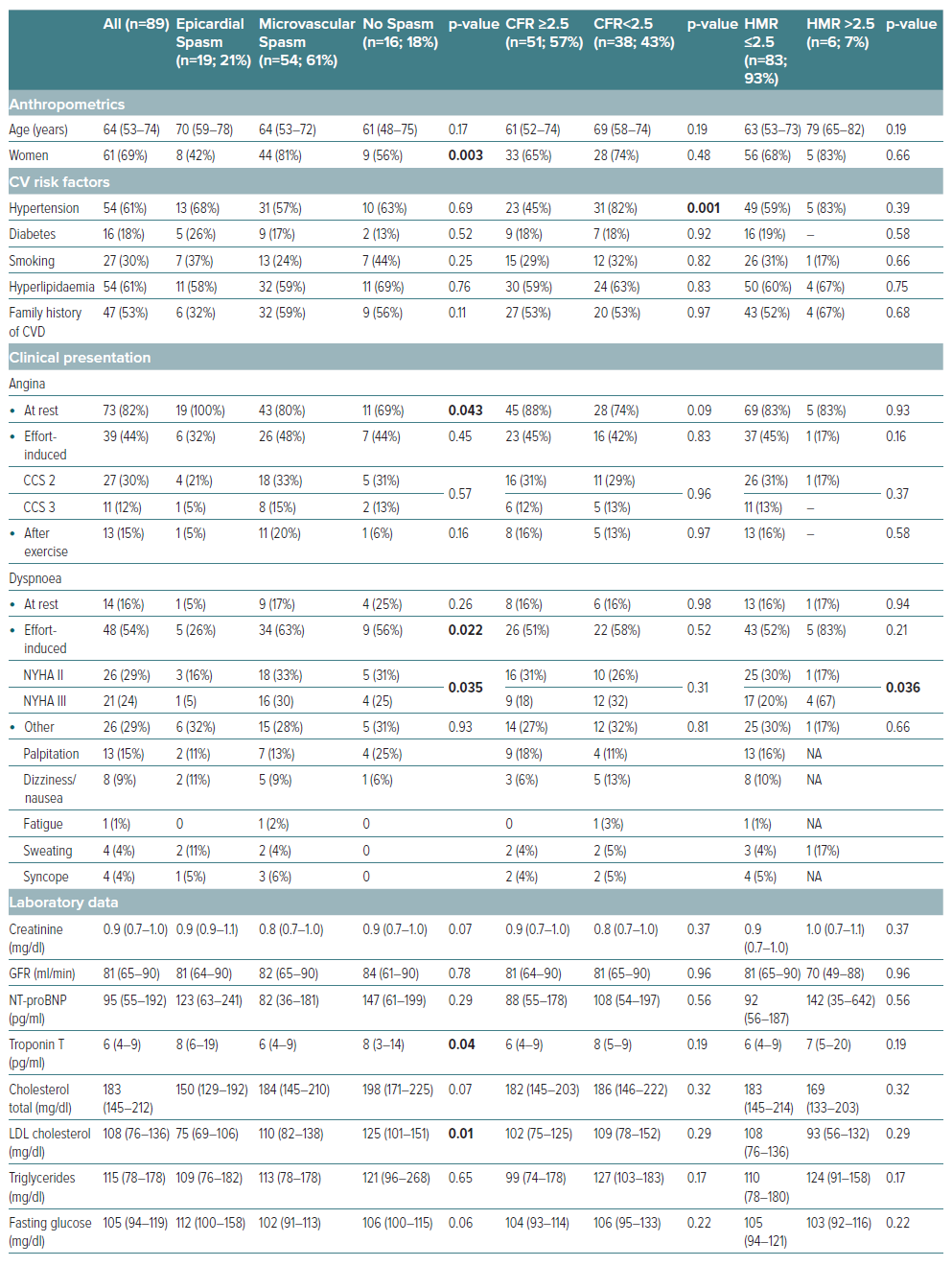
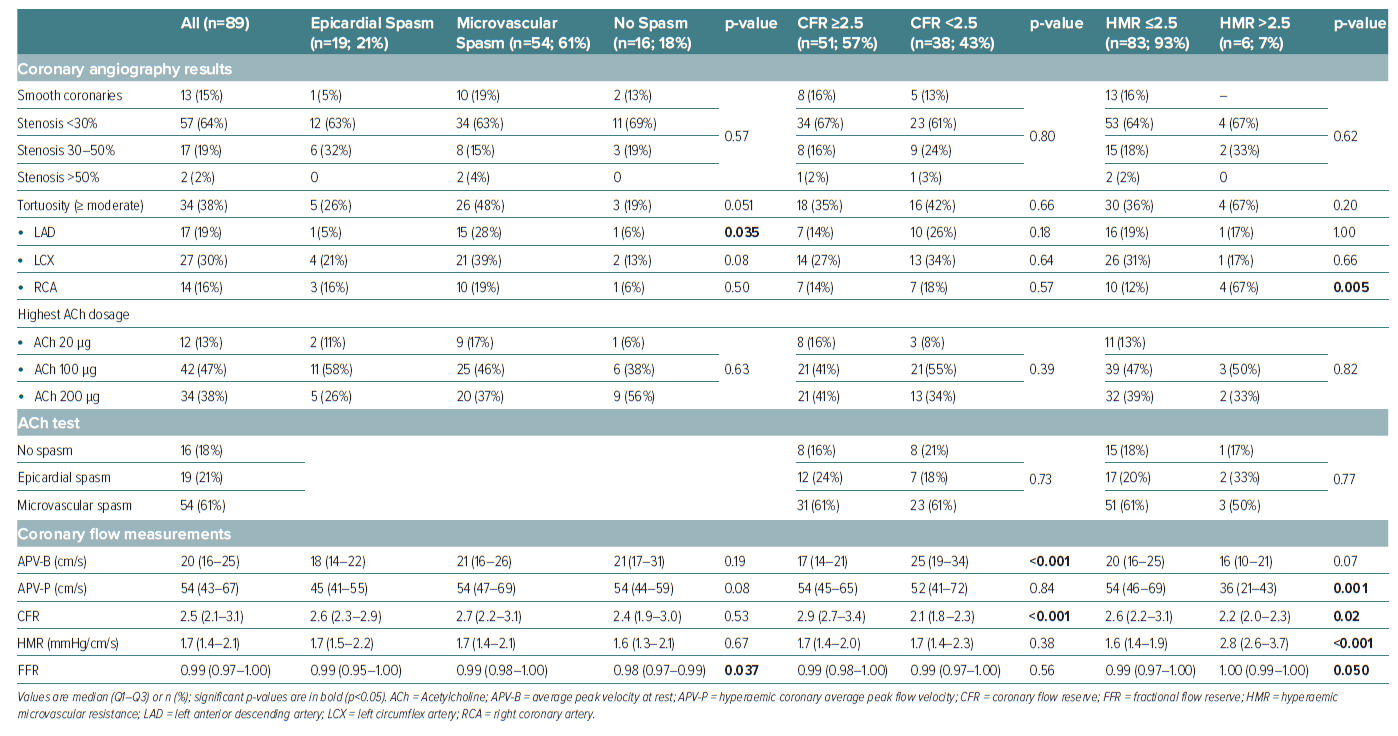
A total of 89 patients with ANOCA were included in this study. The patients had a median age of 64 years (Q1–Q3: 53–74), and 69% (n=61) were women. The baseline characteristics are summarised in Table 1. CFT revealed CFD in most patients (91%) and the frequency of endotypes is illustrated in Supplementary Figure 1. ACh spasm provocation testing exhibited epicardial spasm in 19 (21%) and microvascular spasm in 54 patients (61%). Notably, 16 patients (18%) had a normal ACh test result without symptom reproduction, ECG changes, or significant angiographic vasoconstriction. Among the patients diagnosed with epicardial spasm, eight (42%) presented concurrently with microvascular spasm in response to the preceding ACh dose. Consequently, these individuals could be categorised as having both epicardial and microvascular spasm, leading to an increase to 62 patients with microvascular spasm (70%). However, in Supplementary Figure 1, we opted to depict the distribution of CFT results based on coronary vasomotor responses to the highest ACh dose used according to the study protocol.
Following spasm provocation testing, coronary vasodilatory function was evaluated using a guidewire-based method to measure CFR and HMR. Diminished CFR, defined as CFR <2.5, was observed in 38 patients (43%), while increased HMR (>2.5) was found in six patients (7%), all of whom also had reduced CFR. Only a small proportion (9%, n=8) of patients with abnormal CFT results had isolated CMD without coronary spasm.
There were no significant differences in cardiovascular risk factors among the different endotypes of CFD except for arterial hypertension being more prevalent in patients with reduced CFR. The proportion of women was significantly higher among patients with microvascular spasm (81%, n=44) compared to 42% (n=8) of patients with epicardial spasm and 56% (n=9) of patients without coronary spasm, p=0.003.
Clinical Presentation
There were significant differences in the clinical presentation of angina among patients with different coronary vasomotor disorder endotypes. All patients with epicardial coronary spasm experienced angina at rest, whereas only 80% (n=43) of patients with microvascular spasm and 69% (n=11) of patients without coronary spasm had chest pain at rest (p=0.043). Effort-induced dyspnoea, a potential angina equivalent, also showed significant differences in its distribution. Specifically, 63% (n=34) of patients with microvascular spasm had effort-induced dyspnoea, compared to 26% (n=5) with epicardial coronary spasm and 56% (n=9) without coronary spasm (p=0.022).
Furthermore, we examined the clinical presentation of angina and dyspnoea in patients with CMD compared to those without CMD. Interestingly, there were no overall differences in angina or dyspnoea symptoms between these two groups. However, we observed a significant trend towards more severe effort-induced dyspnoea (NYHA III) among the small subgroup of patients with HMR >2.5 compared to those with HMR ≤2.5 (67% [n=4] versus 21% [n=17], p=0.036).
Coronary Flow Measurements
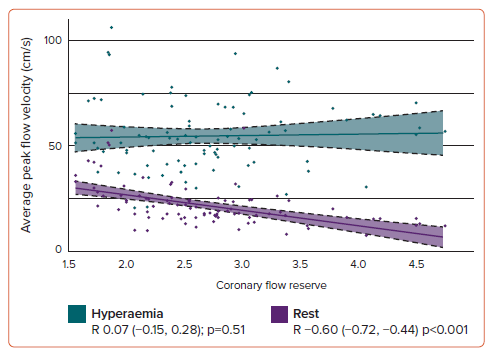
In patients with reduced CFR (<2.5), the median CFR was 2.1 (interquartile range (IQR): 1.8–2.3), and 74% (n=28) of them were women. Patients with increased HMR (>2.5) had a median HMR of 2.8 (IQR: 2.6–3.7), and 83% (n=5) were women. Notably, coronary average peak flow velocity at rest (APV-B) was significantly increased in patients with reduced CFR (25 cm/s [IQR: 19–34]) compared to the group of patients with normal CFR (17 cm/s [IQR: 14–21], p<0.001). Conversely, hyperaemic coronary average peak flow velocity (APV-P) was significantly decreased in the group of patients with increased HMR (36 cm/s IQR: 21–43) compared to those with normal HMR (54 cm/s IQR: 46–69). Notably, while CFR showed a significant correlation with APV-B, no significant correlation was found between CFR and APV-P (Figure 2).
Association of Coronary Tortuosity with Coronary Functional Disease
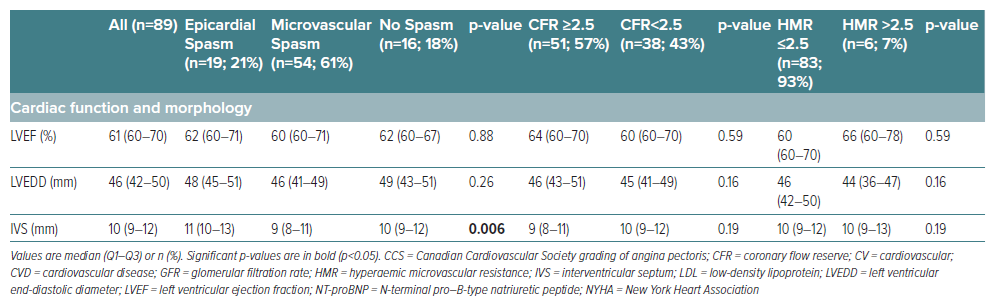
A significant correlation between coronary tortuosity and the outcome of the ACh test was observed (Table 2). Substantially greater tortuosity was identified in cases of microvascular spasm (compared to epicardial spasm patients or those without spasm), primarily influenced by disparities in tortuosity within the LAD (p=0.035). Additionally, a higher prevalence of at least moderate RCA tortuosity was observed in patients demonstrating elevated HMR compared to individuals with normal HMR (p=0.005). Notably, no significant differences regarding tortuosity were found among patients with normal versus diminished CFR. Stratifying patients according to the presence or absence of at least moderate coronary tortuosity revealed a significant association of microvascular spasm with tortuosity (76% versus 51%, p=0.02), whereas no significant differences were observed for epicardial or no spasm as well as CFR and HMR, respectively (Supplementary Table 1). Given that coronary tortuosity is reported in the literature to be associated with arterial hypertension, we investigated whether this association held true in our cohort. Among the 54 patients with arterial hypertension, only 18 patients (33%) displayed at least moderate tortuosity. In contrast, among the 35 patients without arterial hypertension, 16 patients (46%) exhibited at least moderate tortuosity (p=0.27).
Discussion
Our study provides valuable insights into the prevalence and characteristics of CFD in patients with ANOCA (1). We observed a high frequency of coronary vasomotor disorders, with coronary artery spasm being the most prevalent (2). Microvascular spasm was more common than impaired vasodilator capacity (reduced CFR and/or increased HMR) (3). Low CFR was mainly associated with high resting coronary flow (4). Coronary tortuosity is significantly associated with microvascular spasm but not with CMD.
First, we observed a high prevalence of coronary vasomotor disorders in our ANOCA cohort, with 91% (n=81) of patients showing CFD. The remaining 9% (n=8) of our cohort exhibited normal coronary vasomotion despite experiencing chest pain. These patients were considered to have non-ischaemic chest pain, potentially with a musculoskeletal origin. The 91% (n=81) positive CFTs were mainly driven by positive results in the ACh spasm provocation test with 82% (n=73) of ANOCA patients showing epicardial or microvascular coronary spasm. Looking at the distribution of those two endotypes of coronary spasm, 74% (n=54) of our ACh+ patients had microvascular spasm. This prevalence of microvascular spasm is higher than previously reported by Konst et al. who reported roughly a 50/50 distribution of epicardial and microvascular spasm among ANOCA patients with a positive ACh test.10 However, the authors revisited patients who exhibited epicardial spasm in response to the previous ACh dose and found that 33% of these individuals had microvascular spasm at the lower dose, thereby augmenting the overall prevalence of this particular endotype, which was similar to the pattern observed in our cohort.10
Second, CMD (defined as impaired microvascular vasodilatory capacity in response to adenosine) was present in a substantial proportion (43%) of our patients with ANOCA. This finding aligns with existing literature that has shown a high prevalence of CMD in this patient population.5,10,22,23 However, it is worth noting that only 9% of patients with abnormal CFT results had isolated CMD without coronary spasm. Konst et al. reported an even lower number of patients with isolated CMD (only three out of 36 patients having an abnormal adenosine test).10 This indicates that most patients with CMD have a combination of coronary spasm and CMD, underscoring the importance of comprehensive CFT including spasm testing to accurately assess and differentiate these co-existing endotypes. This is crucial as the treatment for coronary spasm differs from that for CMD alone.24 Additionally, an impaired CFR has strong prognostic implications, similar to a positive ACh test result.25,26
In contrast to Konst et al., we used HMR instead of the index of microcirculatory resistance (IMR) to measure microvascular resistance.10 Both methods use adenosine as the hyperaemia-inducing agent. However, in our study, coronary flow measurements were conducted using a Doppler wire, while Konst et al. employed the bolus thermodilution method and reported a higher percentage of patients with elevated IMR (78%, 28 out of 36 CMD patients) compared to the percentage of patients with increased HMR in our study (7%, six out of 38 CMD patients).10 Williams et al. demonstrated that IMR and HMR only moderately correlate, indicating they are not equivalent indices of coronary microvascular resistance.27 However, they found that the correlation between independent invasive and non-invasive measurements of microvascular function was superior with HMR compared to IMR. Additionally, Konst et al. used adenosine intravenously, whereas we administered it intracoronarily.10 Intracoronary adenosine administration has multiple advantages: the hyperaemic response is almost immediate, allowing for quick measurements and adjustments; direct delivery to the coronary artery ensures efficient and effective hyperaemia; and intracoronary administration requires significantly lower doses of adenosine, reducing systemic side-effects and increasing patient tolerance. Therefore, intracoronary adenosine provides a more controlled and predictable hyperaemic response, which can be beneficial for accurate IMR/HMR measurement.28 An alternative hyperaemic agent for intracoronary administration is papaverine. Papaverine induces strong and relatively long-lasting vasodilation but may cause side-effects such as arrhythmias.29,30 Despite this, adenosine is often preferred due to its rapid action and controllability. However, papaverine can be advantageous in situations requiring prolonged hyperaemia or when adenosine is contraindicated.
Third, our study revealed an interesting observation regarding the relationship between CFR and HMR in patients with reduced CFR. Specifically, we found that low CFR in our study population was mainly driven by high resting coronary flow rather than reduced hyperaemic coronary flow as described by other groups.31,32 High resting coronary flow was particularly frequent in patients with low CFR and normal HMR, suggesting a distinct pathophysiology for decreased CFR. Structural microvascular disease is unlikely to explain such a constellation of measurements. Reduced CFR with high HMR which was seen in only a few patients could be explained by structural remodelling of the arterioles.33 Differentiating between these two entities in a classification of endotypes is important, as it may have implications for treatment strategies. Current clinical practice classifies patients based on the presence of CMD without further differentiation between different underlying mechanisms, but our findings suggest that a stratified treatment approach considering these different mechanisms could be beneficial and should be explored in future clinical research. The mechanisms leading to increased coronary resting flow are not yet understood. It can be speculated that this is either due to myocardial inefficiency, uncoupling from coronary blood flow autoregulation, or a combination of these mechanisms.31 Our results emphasise that CFT should also precisely assess resting coronary flow measurements to further differentiate endotypes of impaired coronary vasodilation.
Furthermore, we reaffirmed the findings from our previously published data regarding the association of coronary tortuosity and coronary spasm: an increased frequency of at least moderate coronary tortuosity in the LAD was observed in patients with microvascular spasm.34 Additionally, the present study expanded this finding by the observation that no association of coronary tortuosity with CMD was present. These results partially align with the findings of Jansen et al., as these authors also failed to identify an association between coronary tortuosity and CMD.35 However, in contrast to our results, they did not observe a connection between coronary tortuosity and coronary spasm. This could potentially be explained by Jansen et al. employing a unique statistical approach in their analysis. They explored the prevalence of coronary spasm across various tortuosity groups, whereas our study focused on investigating the prevalence of coronary tortuosity within different groups based on the results of the spasm test. This discrepancy in analytical methodologies may contribute to the differences observed in the findings between the two studies.
The prevailing understanding of the relationship between coronary tortuosity and chest pain rests on the assumption that coronary tortuosity precedes and gives rise to reversible ischaemia-induced blood flow changes.36–38 However, an alternative perspective is conceivable: the fundamental pathogenesis of this association may originate primarily from a microvascular issue, subsequently leading to the development of epicardial coronary tortuosity. Dobrin et al. propose that vessel elongation and tortuosity are influenced by two forces: the traction force and the pressure force within the vessel lumen.39 The combination of these forces results in a total longitudinal force that elongates the vessel, counteracted by a retractive force from the stretched arterial wall, primarily attributed to elastin. Changes in this force equilibrium, such as an age-related reduction in retractive force due to increased collagen or hypertension-induced pressure elevation, contribute to vessel elongation. Alternatively, microvascular spasm presents another clinical scenario. Peripheral vasoconstriction induces intraluminal pressure elevation in proximal vascular segments, potentially leading to long-term vessel elongation and coronary tortuosity. Post-menopausal women who exhibit increased tortuosity, may experience this phenomenon due to reduced elastin and increased collagen deposition associated with the menopause. The presence of microvascular spasm in post-menopausal women further promotes coronary lengthening. Additionally, patients with increased HMR show a higher prevalence of moderate coronary tortuosity, suggesting that distal (microvascular) pressure increase may contribute to vessel elongation.
Study Limitations
Our study has some limitations that warrant consideration. First, this was a single-centre study with a relatively small sample size. Second, the study population was predominantly made up of women, which could limit the generalisability of our results to men with ANOCA. However, while the proportion of women was high in this study, the proportion is consistent with the known sex-based prevalence of angina in the absence of obstructive coronary artery disease.40
We only performed CFT in the left coronary artery. However, the recent work from Rehan et al. showed an additional diagnostic value of testing the right coronary artery as multivessel CFT leads to a heightened prevalence of coronary vasomotor dysfunction.41
We do not yet have follow-up data of our patients; thus, we cannot determine how epicardial or microvascular spasm, CFR, and HMR predict cardiovascular outcomes in this patient population and whether there are differences between sexes as well. However, there is already a substantial body of published data regarding the prognostic value of spasm testing and CMD, respectively.42,43
Conclusion
In patients presenting with chest pain but without significant epicardial stenosis, only one in 10 will have a normal CFT. This indicates a substantial prevalence of CFD in patients with ANOCA, with microvascular spasm emerging as the predominant endotype. Thus, intracoronary ACh provocation testing is essential for precise endotype characterisation. The association of coronary tortuosity with microvascular spasm and of CFR with resting coronary flow, respectively, illustrate the complexity of vasomotor dysfunction, warranting further research for refined diagnostic and therapeutic strategies.
Clinical Perspective
- Given the high prevalence of coronary vasomotor disorders in angina with non-obstructed coronary arteries (ANOCA) patients, comprehensive coronary functional testing, including spasm provocation testing and assessment of microvascular vasodilatory function, should be considered as part of the diagnostic evaluation for this patient population.
- The differentiation between different vasomotor dysfunction endotypes is crucial for tailoring appropriate anti-anginal therapies and accurate prognosis prediction.
- A uniform classification of endotypes would be beneficial for clinical trial planning and may contribute to improved outcomes in ANOCA patients.