Diabetes mellitus (DM) is a group of diseases characterised by metabolic disturbances with increasing prevalence worldwide.1 Individuals with DM present several detrimental micro- and macrovascular complications such as retinopathy, nephropathy, neuropathy, atherosclerosis and coronary heart disease.2,3 Accordingly, efforts for early diagnosis and appropriate management are of ultimate importance.
Despite the emphasis by clinicians in the prompt control of DM several cardiovascular diseases such as hypertension, coronary heart disease, stroke, peripheral vascular disease, etc., have been linked to impaired glucose management.4 Recently, the awareness in the scientific community of the two-way association between DM and heart failure (HF) has steadily increased and has gained research interest.
HF is a syndrome with a complex pathophysiology, several aetiologies and different clinical presentations characterised by high morbidity and mortality.5–7 According to some reports the co-existence of HF and DM is as high as almost 40 %,8 growing the necessity for more in-depth understanding of the common pathophysiological pathways and for effective management of both entities.
As several missing links still exist in the connection between HF and DM we review in this article the most recent data underlying the interaction between them and provide an overview of the most important clinical perspectives.
Diabetes Mellitus and Heart Failure – A Bidirectional Relationship
From Diabetes Mellitus to Heart Failure
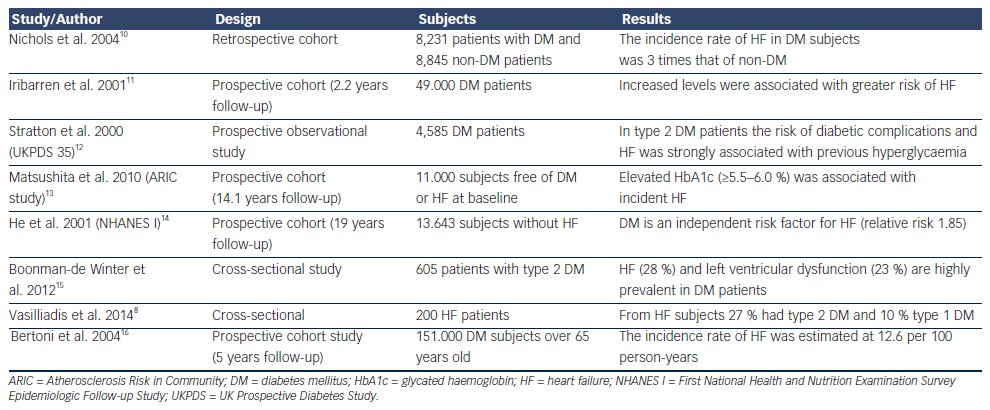
The concept that DM can cause or precipitate HF has been generated even from the Framingham study who estimated that men and women with DM have a two and four times, respectively, increased risk to develop HF compared with non-DM subjects.9 Epidemiological studies have confirmed this relationship and revealed that impaired glucose tolerance, increased serum glucose levels and glycated haemoglobin levels are associated not only with incidence of systolic HF but also with the prevalence of diastolic dysfunction (see Table 1). Accordingly, guidelines have accepted DM and metabolic syndrome as risk factors for HF,17 and the term diabetic cardiomyopathy was used to define either systolic or diastolic left ventricular dysfunction in otherwise healthy diabetic persons in the absence of clinically significant coronary, valvular or hypertensive disease.18 Thus, patients with DM can be categorised in the stage A of the American College of Cardiology Foundation (ACCF)/American Heart Association (AHA) classification of HF, meaning that diabetic subjects are at high-risk for HF but without structural heart disease or symptoms of HF. Therapeutic interventions aim to modify risk factors and guidelines draw attention to monitor and treat DM as early as possible.17
Pathophysiological Connections
Despite the close relationship of these two conditions the difficulties in making a causal pathophysiological connection between DM and HF are formidable as we have to distinguish between insulin deficient and insulin resistant DM and between systolic HF, diastolic HF or HF caused by other aetiologies such as conduction disturbances, tachycardiomyopathy, HF of valvular aetiology, etc. (see Figure 1).
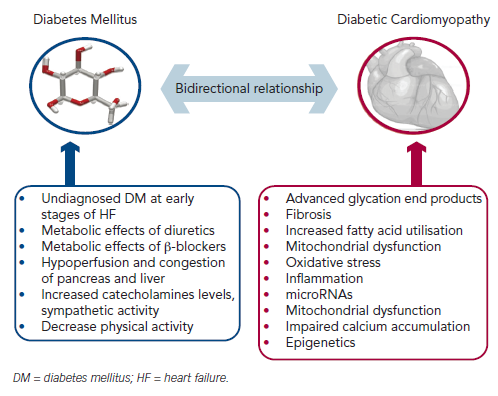
Advanced glycation end products, which are formed in DM subjects after non-enzymatic reaction between protein and sugar residues, can possibly explain the link between DM and HF.19 Advanced glycation end products are increased in patients with chronic HF, they correlate inversely with left ventricular ejection fraction and are related to the severity and prognosis of the disease.20,21 Advanced glycation end products upregulate the hypertrophy-associated genes in cardiomyocytes via the activation of dendritic cells and may be responsible for the hypertrophic and fibrotic phenotype in DM subjects.22
A plethora of other mechanisms have also been proposed. Diabetes-related alterations in the expressions of some calciumassociated proteins may lead to progressive intracellular decay of calcium and in the development of diabetic cardiomyopathy.23 In rats with diabetic cardiomyopathy there are also decreased serum and myocardial levels of adiponectin implying a possible connection between this anti-inflammatory protein and HF.24 Hearts in humans with DM are characterised by increased fatty acid metabolism and oxidation, which is considered a pathophysiological mechanism in the development of HF.25 Moreover, animal studies have revealed increased myocardial levels of cardiotoxic inflammatory cytokines (tumour necrosis factor-alpha, intereukin-6, etc.) in diabetic models.26
Hyperinsulinaemia also impairs phosphatidylinositol 3-kinases pathway and can precipitate myocardial dysfunction.27 Furthermore, accumulation of reactive oxygen species affects the coronary circulation and causes myocardial hypertrophy and fibrosis.28 Carbonic anhydrases have been shown to play a major role in diabetic microangiopathy. Recently, carbonic anhydrases I and II were found elevated in myocardial tissues from post-infarction HF patients with DM. They can induce cardiomyocyte hypertrophy and death in vitro, which are prevented by sodium-hydrogen exchanger-1 inhibition.29 MicroRNAs are also differentially expressed in myocardial tissues from subjects with diabetic cardiomyopathy compared with non-diabetic HF, and specific patterns have been recognised.30–32 These data may provide new targeted treatment of diabetic HF.
We also notice that diabetes frequently precedes coronary heart disease,33 chronic kidney disease34 and hypertension,35 which are major risk factors and account for the majority of HF cases,5 further explaining the close relationship between DM and HF observed in epidemiological studies.
Clinical and Epidemiological Connections
Further to pathophysiological connections, several clinical data have confirmed the detrimental impact of DM in HF course and prognosis. DM predicts readmissions of HF patients36 and increases mortality in subjects with left ventricular dysfunction.37 Elevated troponin levels in patients with DM are also associated with increased HF and cardiovascular mortality.38 Recently, it was also confirmed that DM can be used in a model to predict chronic HF patients at risk of hospitalisation.39 Moreover, in patients with type 2 DM, glycated haemoglobin significantly predicts future HF hospitalisation independently of baseline b-type natriuretic peptide (BNP) level or echocardiographic parameters.40
From Heart Failure to Diabetes Mellitus
The dual nature of the relationship of HF and DM is supported by prospective cohort studies. DM was developed in 29 % of HF subjects compared with 18 % of matched control subjects during a three-year follow-up study.41 Moreover, in a cohort of 50.874 patients, HF severity (as determined by loop diuretic dosages) predicts the risk of developing diabetes after myocardial infarction.42
Nevertheless, the mechanisms precipitating DM and HF are not well-studied. Firstly, we have to notice the possibility that the increased incidence of DM during the course of HF may be an epiphenomenon of the lenient monitoring for impaired glucose metabolism, with glycated haemoglobin and with oral glucose tolerance tests in the early stages of HF. Accordingly, guidelines emphasise the importance of proper diagnosis of DM in HF subjects.5
We can also hypothesise that the decreased physical activity in HF patients may lead to decreased insulin sensitivity and to compensatory insulin requirements and hyperglycaemia. In addition, increased catecholamines levels and sympathetic activity stimulate gluconeogenesis and glycogenolysis.43 Indeed, there is a decrease in insulin sensitivity according to New York Heart Association (NYHA) functional status of HF patients.44
Furthermore we can hypothesise that the haemodynamic consequences accompanying HF (decreased forward blood flow and increased central venous pressure) lead to hypoperfusion and congestion of the pancreas and liver, which may impair their ability to regulate metabolic homeostasis. Confirmatory data are provided by a recent study, which concludes that left ventricular assist devices improve blood glucose control in DM patients.45
Finally, we have to notice the possible adverse effects of established HF treatments, such as beta (β)-blockers and diuretics in blood glucose control.46
Diabetes Mellitus and Heart Failure Diagnosis
As HF diagnosis is based on the combination of clinical data and diagnostic tests, BNP levels can help to distinguish between cardiac and non-cardiac causes of acute dyspnoea in the emergency department.47 BNP levels and reference limits can be affected by several factors such as age, obesity, renal impairment, etc.48 Nevertheless, data from the Breathing Not Properly Multinational Trial suggest that diabetes status is not a confounding variable to be considered when interpreting BNP concentrations in patients who present acutely with dyspnoea.47 However, in asymptomatic diabetic patients there was no significant difference among non-HF patients, HF patients and those with a HF history,49 and no conclusions could be rendered as to the role of BNP testing for screening asymptomatic diabetic patients for left ventricular dysfunction because the degree of disease severity among the diabetic patients could not be assessed. Therefore, in the diagnostic work-up of diabetic patients presented not emergently in primary care centres, we must follow the diagnostic algorithm proposed by the guidelines, which emphasises that patients with high pre-test likelihood of HF may be referred directly for echocardiography.5
Moreover, clinicians should not overlook that DM patients with stable coronary heart disease may present with atypical symptoms, such as shortness of breath. Accordingly, we must be cautious in the interpretation of dyspnoea in these patients.50
Management of Heart Failure and Diabetes Mellitus
As both entities (HF and DM) are characterised by high morbidity and mortality, efforts for the best possible management must be taken. Nevertheless, treatment of HF may adversely interact with DM and vice versa (see Tables 2 and 3).
Heart Failure Treatment – Interaction with Diabetes Mellitus
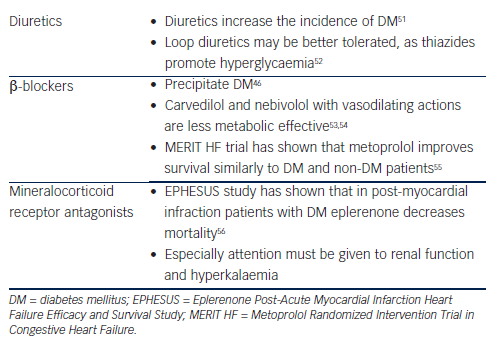
Diuretics
Diuretics, especially furosemide, are one of the most useful treatments in HF patients for symptom relief and are used to achieve euvolaemia.5 Data from studies and meta-analyses (mostly in hypertensive subjects) suggest an association between incident diabetes and diuretics.46 Hypokalaemia, changes in autonomic nervous system function, in beta (β)-cell insulin release and in insulin’s peripheral effects are the proposed mechanisms.51,68 Accordingly, in HF patients under diuretic treatment attention to keep potassium levels and glycaemic status under control must be attained. Thiazides have been shown to promote hyperglycaemia and loop diuretics may be better tolerated in DM patients.52
Beta-blockers
Beta (β)-blockers especially when they are combined with diuretics can precipitate DM.46 Newer β-blockers with vasodilating actions, such as carvedilol and nebivolol, are less metabolic effective compared with metoprolol, do not affect glycaemic control and improve some components of the metabolic syndrome through improvement of the oxidative stress, insulin sensitivity and adiponectin levels.53,54. Vasodilating b-blockers increase survival in HF patients5 and should be preferred in patients with DM. Another β-blocker that can improve survival and symptoms in HF is metoprolol. Although it adversely affects insulin sensitivity, the Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF) trial has shown that it can reduce mortality and HF symptoms similar to DM and non-DM patients.55 A recent prospective study in HF patients with DM also concluded that metoprolol is highly safe and tolerable, and can remarkably improve the clinical status of the patients.69
Mineralocorticoid Receptor Antagonists
Eplerenone is a novel minelarocorticoid receptor antagonist, which is useful in HF patients. Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) in patients after acute myocardial infarction, ejection fraction <40 % and DM revealed that eplerenone on top of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers can decrease mortality.56 As renal dysfunction frequently co-exists with DM and HF,70 attention to renal function and hyperkalaemia must be applied when the combination of eplerenone and angiotensin-converting enzyme inhibitors or angiotensin receptor blockers are used.
Diabetes Mellitus Treatment– Interaction with Heart Failure
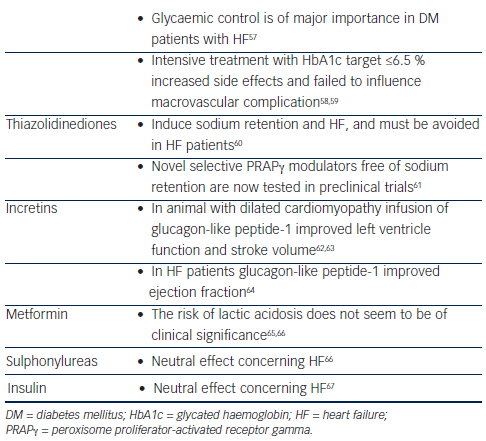
Glycaemic control is of importance in DM subjects with HF as it is supposed to decrease free fatty acid oxidation by myocardial cells and increase the use of glucose as a substrate for metabolic requirements.57 Nevertheless, attention must be given to the results of the Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE) and Action to Control Cardiovascular Risk in Diabetes (ACCORD) trials, which have shown that intensive treatment with a glycated haemoglobin target ≤6.5 % failed to influence macrovascular complications.58,59 The glycaemic control can be achieved with glucose lowering drugs of different categories and it seems that there are differences in the way that they affect HF. Nevertheless, most data are based on observational studies. Randomised control trials exist only for thiazolidinediones.
Thiazolidinediones
Thiazolidinediones are peroxisome proliferator-activated receptor gamma (PRARγ) agonists, which can induce sodium retention and HF. This effect results from the increase in tubular sodium and water reabsorption in specific nephron segments or from stimulation of sodium reabsorption in the collecting duct.60 Although PRARγ remains an attractive target for glycaemic control, thiazolidinediones’ clinical use is now limited and are avoided in patients with HF.71 Nevertheless, several non-thiazolidinedione selective PPARγ modulators free of sodium retention are now tested in preclinical trials.61
Incretins
Glucagon-like peptide-1 (GLP-1) agonists and dipeptidyl peptidase-4 (DPP-4) inhibitors are a new class of anti-diabetic drugs. Except from their favourable effects in glycaemic control, preclinical data and early clinical reports support their clinical benefit in HF patients. GLP-1 increases cyclic adenosine monophosphate in cardiac myocytes but independently from a toxic increase in calcium levels and in myocytes xsxs.72 Preclinical data in dogs and pigs with dilated cardiomyopathy have demonstrated that infusion of GLP-1 improves left ventricle function and stroke volume.62,63 After myocardial infarction infusion of GLP-1, patients with left ventricle dysfunction showed improved ejection fraction and wall motion.73 A non-randomised study in 12 HF patients concluded that chronic infusion of GLP-1 significantly improves left ventricular function, functional status and quality of life in patients with severe HF.64 In contrast, Halbirk et al. concluded that 48 hours infusion of GLP-1 had no major cardiovascular effects in patients without diabetes but with compensated HF.74 Taken together we can conclude that although there are no definite data on the impact of incretins in HF, early observations favour their use in HF subjects.
Other Glucose Lowering Treatment
The main concern regarding the use of metformin in HF patients despite their clinical benefits and the lower rates of mortality was the risk of lactic acidosis.65 Novel data do not support this risk.66,75 UK Prospective Diabetes Study (UKPDS) concluded that there was no difference between sulphonylureas and insulin treatment in cardiovascular complication,66 while it seems that metformin compared with sulphonylureas decrease hospitalisations at least in some studies.66,76 Regarding insulin treatment there are no reports that can affect HF status.67
Non-conventional Treatment of Diabetic Cardiomyopathy
Beyond the standard HF treatment, several novel approaches have been developed (see Table 4).
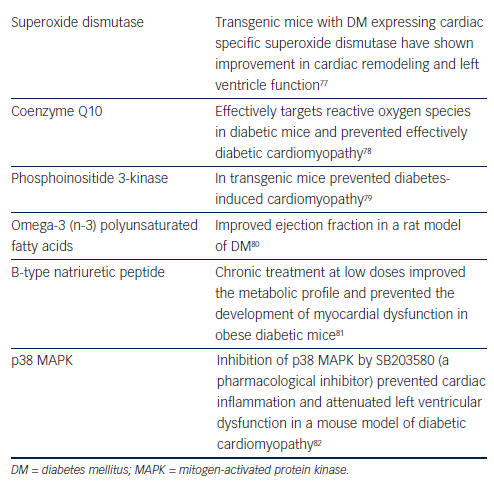
Ex vivo studies support the notion that superoxide dismutase can offer protection from HF.83 In vivo studies in transgenic DM mice expressing cardiac specific superoxide dismutase show improvement in cardiac remodeling and left ventricle function.77
Coenzyme Q10 is a potent antioxidant free of adverse effects, which can maintain cardiomyocyte and mitochondrial function. Recent preclinical data suggest that chronic supplementation with coenzyme Q10 can represent an effective approach for managing diabetic cardiomyopathy.78,84
Phosphoinositide 3-kinase is a cardioprotective kinase, which when enhanced in transgenic mice can prevent diabetes-induced cardiomyopathy adverse cardiac remodeling and dysfunction.79
Interestingly, numerous, mostly preclinical, studies have shown that interventions such as omega-3 (n-3) polyunsaturated fatty acids can improve cardiac output and ejection fraction as well as stroke volume and stroke work in a rat model of DM.80 This improvement may be mediated through attenuation of myocardial connexin-43 abnormalities. In contrast n-6 polyunsaturated fatty acids could accelerate myocardial abnormalities in diabetic rats.85
Conclusion
HF and DM frequently co-exist in a bidirectional relationship as it is proposed by pathophysiological and epidemiological data. At the moment several pathophysiological connections have been proposed but we cannot definitively conclude on the pathophysiological mechanisms precipitating this complex interaction. Both entities are characterised by high morbidity and mortality, and treatment must target the overall improvement as DM treatment can decompensate HF and vice versa. Novel therapeutic agents against diabetic cardiomyopathy are under investigation raising hopes for better management in the future.