Coronary Artery Disease in Patients with Aortic Stenosis
Aortic stenosis (AS) is the most prevalent valve disease in the elderly population, and it is frequently associated with concomitant coronary artery disease (CAD).1,2 The prevalence of CAD in patients with severe AS varies between 15–80% and it is found in about 30% of patients undergoing surgical aortic valve replacement (SAVR).3,4 The degenerative form of heart valve disease and the progression of atherosclerotic disease are similar and share common pathways.5 Epidemiological and histopathological data suggest that degenerative calcific valve heart disease is an active disease process linked to atherosclerosis and elastocalcinosis pathophysiology.1,5
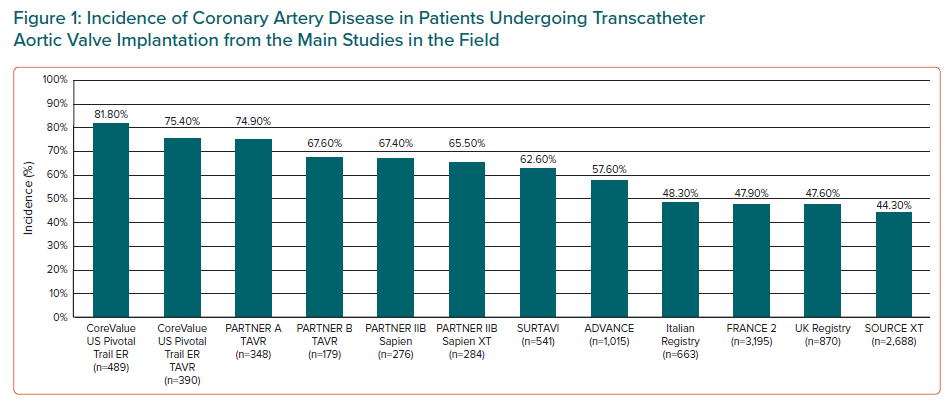
Among patients undergoing transcatheter aortic valve implantation (TAVI), CAD prevalence occurs in 44–81% of patients (Figure 1), many of whom exhibit multivessel disease.6,7 In a recent meta-analysis including 4,000 patients undergoing TAVI with CAD, the mean syntax score (SS) was 14, with the involvement of the left main and left anterior descending artery was 11% and 50% of patients, respectively.8
Observational studies have suggested that there is a reduction of CAD prevalence in low- and intermediate-risk patients when compared to high-risk and inoperable patients.9–15 However, among the whole spectrum of TAVI patients, including those at lower risk, the future development of symptomatic CAD may pose challenges related to coronary access.
Coronary Artery Disease in TAVI Patients
There are many contradictory results reported by observational studies evaluating the clinical outcomes of CAD in patients undergoing TAVI. A systematic review of 15 studies including 8,013 patients undergoing TAVI suggested no significant differences in overall 30-day mortality rates comparing patients with or without CAD. Nonetheless, at 1-year follow-up there was a significant increase in mortality among CAD patients (95% CI [1.07–1.36]; p=0.002).16 D’Ascenzo et al. suggested the risk of death after TAVI may closely convey the complexity of CAD.8 The 1-year mortality rate seems to be higher in patients with an SS >22 and lower in those with an SS <8.
Discrepancies across studies might be explained by the heterogeneity of the definitions used and inclusion criteria. For example, there are different definitions of significant CAD, which may induce positive or negative clinical outcomes depending on severity of coronary lesions, including a lack of objective haemodynamic assessment with fractional flow reserve (FFR) or instantaneous wave-free ratio (iFR), which are rarely used.17 These aspects may also underestimate the completeness of revascularisation and therefore affect clinical outcomes. Likewise, differences among composite clinical endpoints and limited follow-up must also be considered.17
Coronary Artery Disease Assessment
Invasive Coronary Assessment
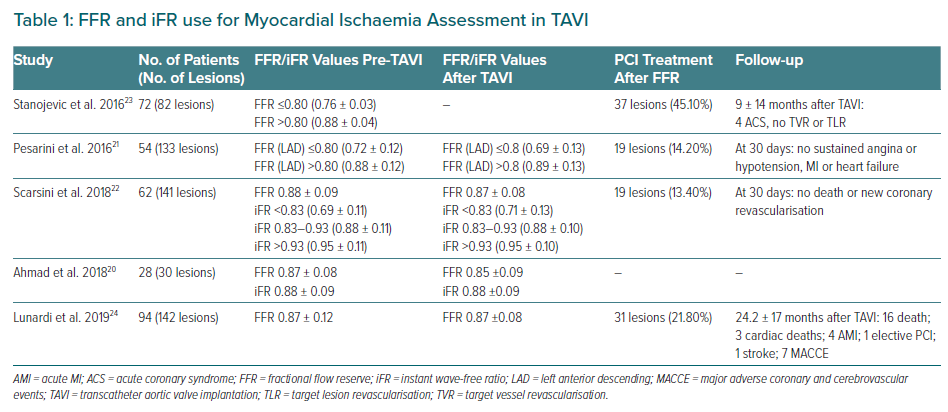
Invasive coronary angiography (ICA) remains the standard method to determine the presence and severity of CAD. However, haemodynamic functional assessment of coronary lesions in TAVI candidates using FFR or iFR may be useful in the presence of a coronary lesion without evidence of ischaemia in the corresponding myocardial territory, once a discrepancy exists between ICA and functional evaluation.18,19 Therefore, the use of FFR is safe and well tolerated in those with severe AS, even using IV adenosine or intracoronary adenosine (Table 1).20–23 A recent study has shown that FFR-guided percutaneous coronary intervention (PCI) in patients with CAD undergoing TAVI yielded lower major adverse cardiac and cerebrovascular event-free survival compared with angiography-guided PCI (92.6% versus 82.0%, respectively; HR 0.4; 95% CI [0.2–1.0]; p=0.035).24 Also, deferred lesions based on FFR presented better outcomes compared with patients who underwent angio-guided PCI (91.4% versus 68.1%, respectively; HR 0.3; 95% CI [0.1–0.6]; p=0.001).24
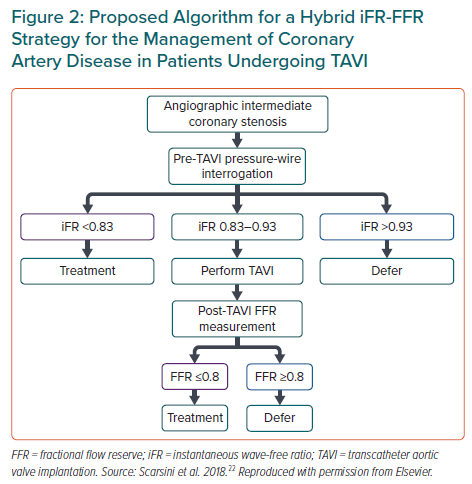
The limitation of FFR use may be the modified coronary flow reserve as a consequence of the left ventricular hypertrophy that is common in AS, resulting in an underestimate of coronary stenosis severity.20 Yet, coronary flow during the wave-free period of diastole has been shown not to change post-TAVI, suggesting that iFR would be less influenced by the effect of the stenotic aortic valve, and would also not need the administration of a vasodilator. It is important to remember that the common threshold of 0.89 for determining lesion severity may not be valid in this population.25 Scarsini et al. proposed a hybrid strategy using FFR only when iFR values were between 0.83 and 0.93, suggesting that 63% of the patients could be assessed without adenosine while maintaining 97% agreement with FFR lesion severity classification (Figure 2).22
Non-invasive Coronary Assessment
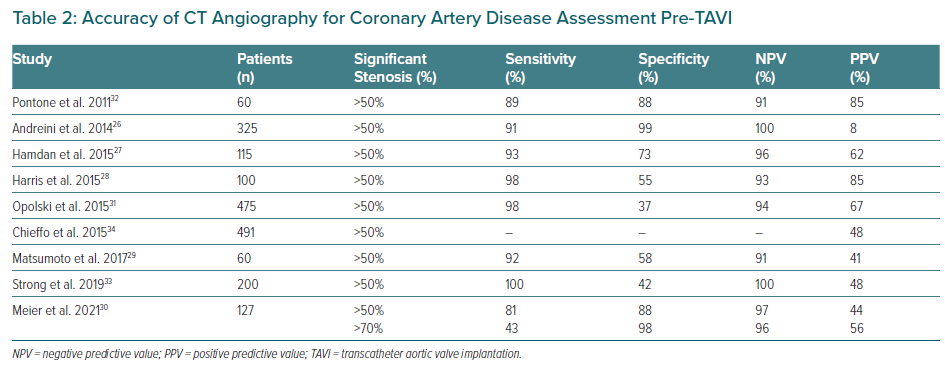
Several studies have compared the performance of CT angiography (CTA) with ICA for the detection of significant coronary stenosis during the pre-TAVI work-up. These studies confirmed a good CTA performance in terms of negative predictive value (NPV), but with a relatively poor specificity (Table 2).26–33
Chieffo et al. showed the feasibility of CTA as a gatekeeper for ICA, with ICA performed in only 24% of TAVI candidates.34 Van Boogert et al. suggested CTA sensitivity, specificity, positive predictive value (PPV) and NPVs of 95, 65, 71 and 94% respectively, for detecting significant stenosis.35,36 Likewise, a recent study including 127 patients compared CTA with ICA images, and an excellent NPV for significant CAD as determined by stenosis ≥50% (97.5%) and for severe CAD with stenosis ≥70% (96.3%) were detected, suggesting a good CTA performance for ruling out CAD pre-TAVI procedure.35 Van den Boogert et al. used the DEPICT CTA database to demonstrate higher CTA diagnostic accuracy to rule out left main (LM) and proximal coronary stenosis in patients undergoing work-up for TAVI, with a sensitivity of 96.4% and NPV of 98.0% for a threshold of ≥50%, and a sensitivity of 96.7% and NPV of 99.0% for a threshold of ≥70% diameter stenosis.36 Therefore, CTA may become an important tool in the stratification of CAD among TAVI candidates, particularly with the increasing number of lower-risk patients.17
Non-invasive methods for diagnosing ischaemic coronary lesions, such as FFR derived from computed tomography angiography (CT-FFR), use computational flow dynamics and provide both anatomical and functional information, ultimately improving the dynamic assessment of patients with CAD.37 In addition, there are promising clinical data with the selective use of CT-FFR in pre-TAVI patients. Michail et al. in the CAST-FFR study suggested a good correlation between diagnostic accuracy of CT-FFR versus conventional FFR in pre-TAVI assessment and an improved accuracy compared to CTA alone.38
Another non-invasive technology for diagnosis of CAD is the angiography-based quantitative flow ratio (QFR) software that uses the Thrombolysis in MI (TIMI) frame count of a single vessel in two orthogonal projections as the surrogate blood flow marker to calculate the translesional gradient ratio. Mejía-Rentería et al. demonstrated superior diagnostic performance of QFR compared to angiography based on FFR as a reference in assessing the functional relevance of coronary lesions in severe AS patients undergoing TAVI (AUC 0.88; 95% CI [0.82–0.93] versus 0.74; 95% CI [0.66–0.81], respectively; p=0.0002)..39 Particularly when the aortic valvular area (AVA) is ≥0.80 cm2, agreement between both methods was 91%, and it decreased to 79% when the AVA was between 0.60 and 0.80 cm, although coronary microvascular dysfunction probably affects the overall diagnostic performance of QFR.39,40
All these promising techniques have some limitations and larger prospective and randomised studies are still warranted.
Percutaneous Coronary
Intervention in TAVI Patients
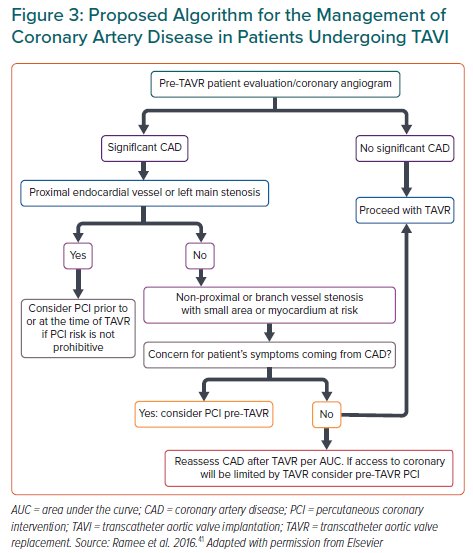
There is a lack of robust data and randomised clinical evidence on the indication for PCI in patients with significant CAD undergoing TAVI. In general, the decision to perform PCI before TAVI commonly relies on recommendations from stable CAD guidelines, including the presence of ischaemic symptoms and the anatomy of the coronary lesions. The American College of Cardiology has proposed a treatment algorithm considering a higher benefit of PCI in patients undergoing TAVI with proximal epicardial coronary stenosis >70% or left main stenosis >50%, patients’ symptoms or if CAD access in the future would be limited by TAVI (Figure 3).41
A previous systematic review of nine studies involving 3,858 participants showed that those patients who underwent PCI showed higher rates of major vascular complications and mortality at 30 days.7 Nonetheless, there were no differences in effect estimates for 30-day cardiovascular mortality, MI, acute kidney injury, stroke or mortality at 1 year.7 The only prospective and randomised controlled trial comparing elective pre-procedure PCI versus no PCI in patients undergoing TAVI with significant CAD is the ACTIVATION trial.42 The study randomised 235 patients to the treatment of significant CAD by PCI (test arm) versus no PCI (control arm). Significant coronary disease was defined as ≥1 lesion of ≥70% severity in a major epicardial vessel or 50% in a vein graft or protected left main lesion.42 There were no differences in the primary endpoints of death or rehospitalisation at 1-year follow-up between the two treatment strategies. However, there was a higher rate of bleeding in the PCI group (44.5% versus 28.4%, p=0.02) in the first 30 days after the TAVI procedure.43
Likewise, Chakravarty et al. evaluated 204 patients with coexisting aortic valve disease and LM coronary disease undergoing TAVI in addition to PCI.44 This large case series demonstrated that even for LM disease in patients undergoing TAVI, it is feasible and safe to perform a planned LM PCI before or during TAVI procedures, with clinical outcomes comparable to that of isolated TAVI procedures.44
Another important aspect in such patients is the completeness of revascularisation when indicated. Using the residual SYNTAX score (rSS), a previous meta-analysis has shown that patients with a higher rSS have increased mortality after TAVI, suggesting the negative impact of incomplete coronary revascularisation.8,45 Although these findings are contradictory and should be evaluated in the context of the presence of ischaemia, it is important to provide complete revascularisation in such patients undergoing TAVI, at least in the main vessels/territories.46
Percutaneous Coronary Intervention Before TAVI
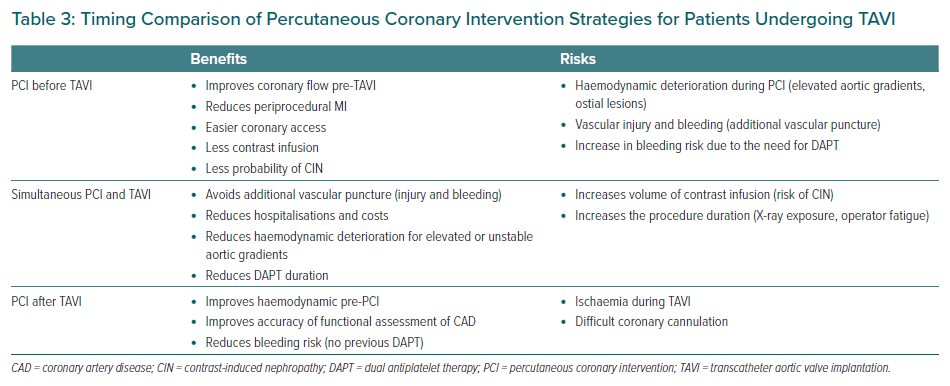
Patients may benefit from both therapeutic strategies, either staged versus simultaneous procedures. Some observational studies have suggested that PCI performed 30 days before TAVI may increase the risk of vascular and bleeding complications, probably associated with the additional vascular puncture.47 However, this strategy may reduce periprocedural MI as well as protecting patients at high-risk for developing contrast-induced nephropathy from dye overload. This is of greater importance for those with previous chronic kidney disease (30–50% of the TAVI population), congestive heart failure, anaemia, AF, among other factors that together may also increase mortality.48
Collectively, considering the absence of robust data and despite some apparently contradictory results, PCI seems to be safe before TAVI procedures in patients with severe CAD who are symptomatic (angina), or have acute coronary syndrome (ACS), or even have positive ischaemia tests (invasive or non-invasive), without a significant increase in the risk of complications with an improvement in the overall prognosis in selected cases after medium- to long-term follow-up.49
Concomitant Percutaneous Coronary Intervention and TAVI
It is possible that simultaneous PCI and TAVI could be an adequate strategy for unstable patients with very elevated aortic gradients who are at high risk for coronary occlusion, such as ostial lesions, low LM height and valve-in-valve (VIV) implant.49 Several studies have suggested the safety of performing PCI and TAVI at the same time, probably due to lower vascular bleeding complications, as well as reducing procedure time and minimising the need for dual antiplatelet therapy (DAPT).50,51
Yang et al. compared concomitant versus staged PCI and TAVI through a systematic review of four studies including 209 patients and showed no differences in overall 30-day mortality between the groups, as well as renal failure, peri-procedural MI, life-threatening bleeding and major stroke.6 Ochiai et al. investigated the optimal timing for PCI comparing three strategies (PCI pre-TAVI versus PCI concomitant to TAVI versus PCI post-TAVI) and found no differences after 2-year follow-up in the composite of major adverse cardiac and cerebrovascular events (concomitant versus pre-TAVI, HR 0.92; 95% CI [0.52–1.66]; p=0.79; post- versus pre-TAVI, HR 0.45; 95% CI [0.18– 1.16]; p=0.10).52 Therefore, the optimal timing of PCI and TAVI remains unclear and must be individualised for each patient (Table 3).
Percutaneous Coronary Intervention After TAVI
As the TAVI procedure become available to patients from all surgical risk categories, a longer life expectancy brings greater possibility of PCI after TAVI, as well as VIV procedures. However, there are a large number of technical difficulties for selective catheterisation of the coronary ostia when the TAVI bioprosthesis is in place and during VIV procedures where it might be unfeasible in approximately a third of patients.53,54
Optimising transcatheter aortic valve commissural alignment in native annulus may avoid coronary artery overlap and facilitate coronary access in the future, especially using certain types of self-expandable transcatheter bioprostheses (Evolut from Medtronic and ACURATE-neo from Boston Scientific).52–56 Redondo et al. developed a promising method for proper commissural alignment (ACURATE-neo bioprosthesis) to insert the delivery system with a patient-specific rotation based on CT data analysis, tested in 3D, printed in silico models and in vivo.56 The results suggested an important reduction in commissural misalignment and coronary artery overlap.56
Yudi et al. proposed a catheter selection algorithm depending on the type of bioprosthesis, the procedure (ICA or PCI), and the position of the transcatheter valve commissure with respect to the coronary ostium.57 Another study reported technical success in all 46 cases of PCI performed immediately after balloon-expandable TAVI within the same operative session.54
Coronary Complications Post-TAVI
Coronary obstruction remains a rare but potentially life-threatening complication of TAVI and it usually occurs after valve implantation and more frequently in women and during VIV procedures.58–60 A low coronary ostium take off and shallow sinus of Valsalva are important risk factors during native aortic valve TAVI. In cases of VIV, the main risk factors relate to the type of surgical bioprosthesis (stented with externally mounted leaflets and stentless bioprosthesis) and a shorter virtual valve-to-coronary ostia distance.58–60 Persistent severe hypotension immediately after valve implantation, irrespective of the presence or absence of ST-segment changes, should prompt the possibility of coronary obstruction, with the evaluation of new segmental abnormalities in the echocardiogram, as well as coronary ostia flow. PCI is a feasible and effective treatment in most cases, although the rates of additional haemodynamic support, conversion to open heart surgery or stent compression requiring the implantation of a second stent remain important challenges. Therefore, preventive measures may also be undertaken, such as using a guidewire, with or without a stent placement, in higher risk patients.
Unplanned PCI following TAVI is not common and ACS is frequently related to an atherothrombotic mechanism, progression of CAD, or failure of a previous PCI. Stefanini et al.’s study, based on multicentre international registry data, reported PCI due to ACS after TAVI at a median time of 191 days.61 There was PCI success in 96.6%, and no significant differences between patients treated with balloon-expandable and self-expandable bioprostheses (100% versus 94.9%; p=0.150).61 However, considering a retrospective cohort study, statistical analysis limitations and significant biases may affect the conclusion. Another recent cohort of 779 patients reported an incidence of ~10% of an ACS at a median follow-up of ~2 years.62 Of note, a non–ST-segment elevation MI (NSTEMI) type 2 occurred in 36%, unstable angina in 35% and NSTEMI type 1 in 28%, yielding an all-cause mortality rate of 37% after a median follow-up of 21 months post-ACS.62
Other potential mechanisms for coronary events might be impaired flow dynamics and coronary hypoperfusion related to the TAVI bioprosthesis, a coronary embolism related to subclinical leaflet thrombosis in a bioprosthetic aortic valve thrombosis, a late valve migration occluding the coronary ostia and Kounis hypersensitivity-associated thrombotic syndrome.62–65
Dual Anti-platelet Therapy Timing
Medical therapy including antiplatelet therapy is often recommended for asymptomatic patients with ACS or stable CAD, with applicability for the TAVI population. Nonetheless, safety of DAPT is an important consideration for PCI in TAVI patients given the higher bleeding risk (HBR) profile that is generally seen among these patients. Although more potent antiplatelet therapy with ticagrelor and prasugrel may be considered for high-risk PCI, especially in patients with a recent ACS, there are no data evaluating its safety in such HBR patients, especially in the periprocedural timing of the TAVI procedure.18,19
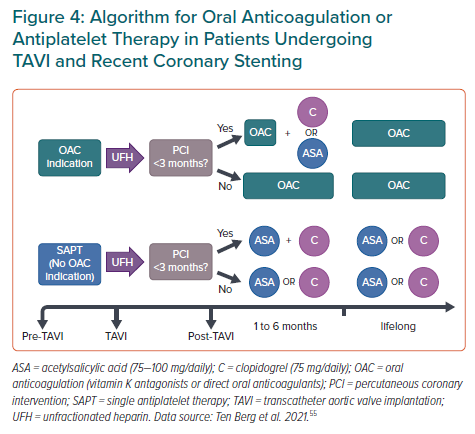
Rodes-Cabau et al. suggested a tendency of DAPT (aspirin plus clopidogrel) to increase the incidence of death, MI, ischaemic stroke or transient ischaemic attack, or major or life-threatening bleeding events compared with single antiplatelet therapy (aspirin) at 3-month follow-up. The POPular TAVI trial showed similar results at 1-year follow-up in patients without indication for long-term oral anticoagulation.66,67 Thus, the combination of low-dose aspirin plus clopidogrel should be recommended after PCI with therapy duration between 3 to 6 months.18,19 However, after this period and for those patients without CAD undergoing TAVI, monotherapy either with low-dose aspirin or clopidogrel is recommended (Figure 4).55,68
Finally, among patients undergoing TAVI more than 35% have concomitant AF with an indication for oral anticoagulation (OAC) and without recent PCI or formal indication to receive antiplatelet therapy.69 OAC alone should be indicated in such circumstances (Figure 4).55,68 In patients with an indication for concomitant use of OAC and antiplatelet therapy, triple therapy (DAPT plus OAC) should be avoided, and only suggested for those patients with a very high thrombotic risk but restricted to a very short period of time (usually for 1 month after recent PCI), followed by clopidogrel alone plus OAC.70,71
Conclusion
CAD concomitant with severe AS in elderly patients undergoing TAVI is very common and therefore requires careful assessment and proper management of ischaemia. Although the standard evaluation of coronary anatomy is still invasive ICA, complementary haemodynamic functional methods such as FFR and iFR and non-invasive methods, such as CTA and FFR-CTA, may improve the diagnosis of ischaemia in such patients and decrease the probability of unnecessary PCI.
Since the prognosis of pre-emptive PCI in patients undergoing TAVI with CAD is uncertain and the optimal timing for PCI is still controversial, an individualised approach should be based on the patient’s profile, characteristics, symptoms and preferences, as well as the anatomical feasibility of the procedure. Future studies with a larger number of patients are of utmost importance to better define the best treatment of patients with concomitant CAD undergoing TAVI.