Despite improvements in its prevention and management, coronary artery disease (CAD) remains a major cause of death in the developed world and is responsible for more than four million deaths in Europe each year.1 Acute coronary syndrome (ACS) is the most severe clinical manifestation of CAD and includes unstable angina, non-ST-elevation MI (NSTEMI) and ST-elevation MI (STEMI).
Although treatment of ACS has progressed considerably, approximately 20% of ACS survivors still experience a subsequent ischaemic cardiovascular event within 24 months and 5-year mortality ranges from 19% to 22%, indicating a missed opportunity for incremental benefit.2,3
Acute Coronary Syndrome
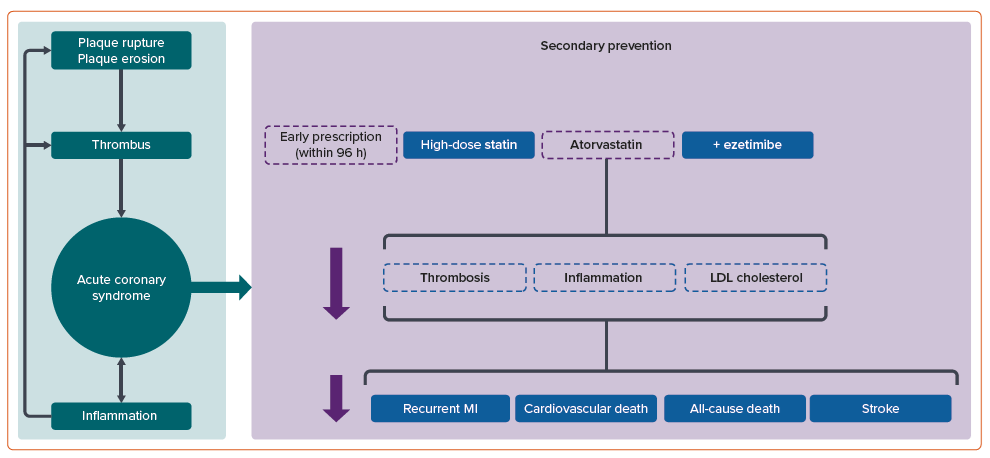
ACS typically results from plaque rupture or erosion within the coronary arteries, instigating thrombus formation and ensuing myocardial ischaemia or MI.4,5 Despite progress in antiplatelet therapy and advances in percutaneous coronary intervention (PCI) and coronary artery bypass grafting, patients with ACS are still exposed to a significant residual risk of recurrent MI and premature mortality.6,7 This highlights the need to explore additional risk reduction strategies for this population.
Secondary prevention, which focuses on managing modifiable risk factors and employing evidence-based treatments, is essential in minimising the likelihood of future cardiovascular events and improving patient outcomes after an ACS event (Figure 1).8
Lipid-lowering Targets
LDL cholesterol levels tend to decrease during the first few days following ACS, so a full, non-fasting blood lipid profile should be obtained as soon as possible after admission.9
European Society of Cardiology (ESC) guidelines recommend LDL cholesterol should be lowered to <1.4 mmol/l in ACS patients and, in those who develop a further ischaemic event within 2 years of their index event, stricter target LDL cholesterol levels of <1.0 mmol/l.10 These differ from the National Institute of Health and Care Excellence (NICE) guideline target of 2.0 mmol/l in secondary prevention patients.11 The American Heart Association and American College of Cardiologists jointly recommend combination therapy of statins with a non-statin medication to achieve target LDL cholesterol levels of <1.8 mmol/l in high-risk patients.12
The fifth European survey of cardiovascular disease prevention and diabetes (EUROASPIRE V) survey reported that only 30% of post-ACS patients achieved LDL cholesterol levels <1.8 mmol/l 1 year after discharge, while the ACS EuroPath survey revealed a considerable lack of physicians’ compliance with guidelines in lipid lowering in post-ACS patients.13,14
Lower LDL cholesterol levels are correlated with a reduction in ongoing risk. A post-hoc analysis of the TNT study found a significant reduction in the risk of major adverse cardiovascular events (MACEs; all-cause mortality and cardiovascular death) with falling LDL cholesterol levels.15 Results from pivotal trials are consistent with “the lower, the better” approach to lowering LDL cholesterol in patients with atherosclerotic cardiovascular death (ASCVD).16
Statin Treatment
Statin therapy improves cardiovascular outcomes in ACS patients through modulation of several pathophysiological processes beyond lipid lowering.17
Atherosclerosis is a chronic inflammatory condition where there are dysfunctional interactions between immune cells and vascular endothelium; 60% of ACS patients have an elevated level of high-sensitivity C-reactive protein (hsCRP), a biomarker of systemic inflammation.18,19 Data support potential anti-inflammatory effects of statins in both primary and secondary prevention clinical trials with demonstrable reductions in C-reactive protein (CRP).20 Statins are thought to modulate thrombogenesis through a reduction in tissue factor expression as well as attenuation of endothelial dysfunction that occurs in acute plaque rupture, albeit in animal models of atherosclerosis.21,22
Statins exhibit a dose-dependent effect on intimal hyperplasia, ventricular hypertrophy and platelet activation, specifically interacting with platelet receptors; they reduce adhesion, aggregation, degranulation, inflammation, vasoconstriction and oxidative stress. These mechanisms may account for the anti-thrombotic effect associated with statin therapy, which is enhanced by ezetimibe.23
MIRACL was the first randomised controlled double-blind trial exploring the potential benefits of early versus late statin prescription in unstable patients (defined as those presenting with unstable angina or non-Q wave MI). In this trial, 3,086 patients were randomised to early prescription (24–96 hours following admission) of high-dose atorvastatin 80 mg/day versus placebo for 16 weeks; there was a significant reduction in MACE in the atorvastatin arm.24 Despite the relatively small sample size and short follow-up period of 16 weeks, a greater absolute risk reduction was demonstrated in this short time frame when compared with trials in stable CAD.
Early data supporting statin use in ACS patients were demonstrated in the RIKS-HIA study, where 5,528 patients who received statins by the time of discharge were shown to have a significantly lower 1-year mortality rate than 14,071 patients who did not.25 Furthermore, a pooled analysis from the GUSTO-IIb and PURSUIT trials of 20,809 patients demonstrated a survival benefit in ACS patients discharged on a statin after propensity matching.26
In the CALLINICUS-Hellas registry, 780 consecutive patients with ACS were recruited, nearly half of whom exhibited high-risk characteristics and were eligible for in-hospital triple lipid-lowering therapy (LLT), including a statin, ezetimibe and a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor, with the goal of achieving an LDL cholesterol level <1.04 mmol/l.27
PROVE IT-TIMI 22 recruited 4,162 ACS patients who had experienced an MI within the previous 10 days and had a total cholesterol of ≤6.21 mmol/l within the first 24 hours of admission or ≤5.18 mmol/l in those already on lipid-lowering therapy.28 Patients were randomised to receive either intensive therapy with atorvastatin 80 mg/day or pravastatin 40 mg/day; only 69% had undergone revascularisation for the index presentation.
Intensive atorvastatin therapy resulted in a median reduction of LDL cholesterol (2.46 mmol/l versus 1.60 mmol/l) and a reduction in the first occurrence of primary endpoints (death, MI, unstable angina requiring hospitalisation, stroke and revascularisation at ≥30 days) by 16% and subsequent events by 19% at 2-year follow-up. A significant reduction in CRP was also seen in both groups, although this was significantly greater in the atorvastatin treated patients, accompanied by a rise in HDL cholesterol in both groups.28
Although not statistically significant, a trend towards reduction in death from any cause or MACE was noted after 30 days of enrolment, achieving statistical significance after 180 days.28 The authors conceded that while the reduction in clinical events correlated with LDL cholesterol reduction, as seen in the Heart Protection Study where a 1.03 mmol/l reduction in LDL cholesterol correlated with a 25% reduction in cardiovascular events compared to placebo; the non-lipid-lowering potential pleiotropic effects of atorvastatin may have contributed to this.29,30
Ezetimibe
Ezetimibe is a cholesterol absorption inhibitor that inhibits intestinal uptake of dietary and biliary cholesterol and can reduce LDL cholesterol by 15–22% when used as monotherapy, although there is significant variability between individuals.31
In the pivotal IMPROVE-IT study of patients with recent ACS, the addition of ezetimibe to maximal dose simvastatin led to a further 17–23% reduction in LDL cholesterol and a significant albeit limited reduction in both composite primary and secondary endpoints (risk of cardiovascular death, major coronary event or non-fatal stroke) with high-risk patients appearing to benefit more.32,33 Specifically, patients with diabetes and prior coronary artery bypass graft surgery were noted to have a greater reduction in the primary composite endpoint following ezetimibe addition.33
Targeting adjunctive ezetimibe therapy may be of particular use in high-risk patients, such as people aged >75 years or those with raised biomarkers of significance (high-sensitivity troponin, hsCRP, N-terminal pro-B-type natriuretic peptide) or reduced estimated glomerular filtration rate.34 As a consequence of IMPROVE-IT, real-world registries have noted an increase in ezetimibe use in clinical practice among patients treated for ACS, resulting in improved LDL cholesterol target achievement.35
A recent systematic literature review and meta-analysis demonstrated that patients with ASCVD receiving combination ezetimibe plus statin therapy experienced an additional 0.57 mmol/l reduction in LDL cholesterol compared with patients receiving statin therapy alone. This modest incremental reduction in LDL cholesterol suggests that patients with ASCVD with LDL cholesterol levels >0.52–0.65 mmol/l above the desired target despite statin therapy may need additional lipid-lowering therapy options such as PCSK9 inhibitors.36
These data have contributed to ezetimibe receiving a Class 1 (level of evidence B) recommendation in recent ESC/European Atherosclerosis Society guidelines and it is also recommended by NICE, albeit based largely on data for primary prevention.10,11 Similarly, corresponding US guidelines recommend ezetimibe to varying degrees depending upon individualised risk stratification as a statin adjunct, with higher degrees of recommendation in those at highest risk of ASCVD.12
In a pilot study of 248 patients, nearly half did not reach the LDL cholesterol targets recommended by guidelines within 1 year of follow-up, despite the majority having already been prescribed high-dose statins following an ACS event. Additional adjunctive treatments besides statins were not prescribed sufficiently for this high-risk group, which may be due to gaps in physician knowledge, reluctance to change treatment, patient rejection or concerns over cost and insurance coverage.37
A meta-analysis of 11 studies including more than 20,000 patients and a systemic review of 4,561 articles demonstrated that the addition of ezetimibe to high-dose statin therapy during the initial ACS event leads to significant improvements in reducing LDL cholesterol and total cholesterol levels at day 7, 1 month, 3 months and 1 year after the event. This results in a significant decrease in recurrent cardiovascular events, including major ACS incidents, non-fatal strokes, non-fatal heart attacks and ischaemic strokes, as well as mortality from any cause following an ACS episode.38
The PENELOPE study found that a gradual approach to lowering lipid levels using statins and/or ezetimibe led to 84% of post-ACS patients categorised as very high-risk achieving an LDL cholesterol level of ≤1.8 mmol/l.39 This straightforward and cost-effective method has the potential to attain guideline-directed lipid targets.
A more recent retrospective study with 768 patients in each group found that starting ACS patients with an upfront combination therapy of statin and ezetimibe reduced all-cause mortality when compared to statin monotherapy alone.40 These findings indicate that, in high-risk patients, combination therapy upfront should be favoured over a stepwise approach of additive LLT.
Subgroup analysis from IMPROVE IT revealed that further LDL cholesterol reduction with ezetimibe in addition to statin therapy in ACS patients was linked to a decreased risk of peripheral vascular adverse events over long-term follow-up, such as critical limb ischaemia and progressive disease requiring revascularisation, emphasising the additional LLT benefits in patients with peripheral artery disease.41
Pre-procedural Statin Loading
Several small studies in ACS have been conducted in patients with NSTEMI and demonstrated that statin treatment prior to PCI reduced the incidence of MACE and/or post-PCI elevation in levels of myocardial injury and inflammatory markers.42
Contemporary guidelines recommend routine pre-treatment or loading against a background of long-term therapy with a high-dose statin in patients undergoing urgent/emergent PCI for ACS or elective PCI.10 A meta-analysis of 13 randomised studies including 3,341 patients demonstrated a reduced risk of both periprocedural MI and 30-day MACE by 44% in high-dose statin pre-treated patients, although most of these studies included patients with stable angina undergoing elective PCI.43
The ISCAP trial compared intensive statin treatment with usual care in 1,202 patients with stable angina or NSTEMI undergoing PCI and found similar 30-day MACE rates (cardiac death, MI or unexpected target vessel revascularisation) between the two groups, indicating that serial intensive statin regimens did not improve clinical outcomes in patients undergoing elective PCI; however, only 25% of trial participants included were NSTEMI patients with both high-risk NSTEMI and STEMI patients excluded.44
The more recent SECURE-PCI trial included 4,191 patients with ACS (both NSTEMI and STEMI patients) with planned invasive management randomised to periprocedural statin loading or placebo. At 30 days, MACEs were recorded in 6.2% of the loading dose group and in 7.1% of the placebo group, a difference that was not statistically significant.45 These two large trials do not support routine statin loading doses in patients with ACS undergoing PCI but rather suggest continuous, high-intensity statin therapy may be sufficient to achieve improved clinical outcomes in these patients.46
The Swedish nationwide cohort study, tracking 40,607 patients, offers compelling evidence to support early and significant decrease in LDL cholesterol levels after MI.47 Those with a greater LDL cholesterol reduction had notably lower risk ratios for a range of outcomes including cardiovascular mortality, MI and ischaemic stroke, as well as all-cause mortality and hospitalisation due to heart failure. Similarly, other studies have demonstrated improved cardiovascular outcomes with early statin prescription in patients presenting with ACS.48–52
Borovac et al. conducted a meta-analysis evaluating the efficacy of early high-dose loading of atorvastatin and rosuvastatin in reducing major adverse cardiovascular and cerebrovascular events (MACCEs) and recurrent MI among statin-naive patients with ACS undergoing planned PCI.50 From 11 trials including 6,291 patients, they found that high-dose statin loading prior to PCI significantly reduced the incidence of MACCEs and recurrent MI during the short-term period after PCI (within 30 days). This effect was more pronounced in patients with STEMI when loaded with high-dose atorvastatin, while non-ST-elevation ACS patients loaded with high-dose rosuvastatin also benefited significantly.
High-dose statin loading using atorvastatin or rosuvastatin before revascularisation for STEMI and NSTEMI was also found to reduce the rate of overall microvascular obstruction at the capillary bed of the coronary arteries.52
Secondary Prevention
Following ACS, mortality rates remain high at 15% and 18% for patients in Belgium and the UK, respectively.53 Patients with NSTEMI were found to have a poorer prognosis at 6-month follow-up compared to STEMI patients, which may be due to suboptimal LLT and incomplete revascularisation.
Medication compliance among patients is highest in the first month after ACS. Cheng et al. reported that, of patients discharged on aspirin, β-blockers and statins, 34% had stopped at least one medicine and 12% had stopped all three medications a month after ACS. Only 40–45% of patients remained adherent with β-blocker or statin therapy 1–2 years following ACS, highlighting the importance of cogent secondary prevention planning, particularly in high-risk patients.54
Statins in Secondary Prevention
Several large randomised controlled trials have demonstrated the benefits of prescribing simvastatin, pravastatin, lovastatin, fluvastatin and atorvastatin to patients with angina or prior MI when compared with placebo, significantly reducing the incidence of MACEs.55–59
High-dose atorvastatin at 80 mg/day was shown to benefit patients with a definite history of prior MI in the IDEAL study, compared to simvastatin 20 mg/day. Despite it not reaching its composite primary outcome, there was a reduction in non-fatal MI and composite secondary outcome largely driven by a reduction in the need for coronary revascularisation.60 Further subgroup analysis revealed that patients who achieved LDL cholesterol of <1.55 mmol/l experienced fewer major cardiac events, suggesting benefits of high-dose atorvastatin prescription and strict LDL cholesterol targets.61 Similarly, the TNT study randomised patients with stable CAD to high-dose (80 mg/day) or low-dose (10 mg/day) atorvastatin and found a reduction in both LDL cholesterol and MACE rates with similar rates of statin-related myalgias noted.62
Only a proportion of patients discussed in these trials had prior MI. Although IDEAL mandated prior MI in its inclusion criteria, only 11% had experienced acute MI within the 2 months before recruitment, a study of the effectiveness of additional reductions in cholesterol and homocysteine (SEARCH) did not disclose the time frame of prior MI and, in TNT, 58% of patients had experienced a prior MI.60,62,63 Therefore, these trials do not accurately reflect an ACS population but do underline the importance of the ongoing need to treat to lipid targets for improved outcome.
Long-term Outcomes
Several secondary prevention trials have conducted long-term follow-up of mortality and morbidity outcomes (11-year follow-up in the Heart Protection Study; 10.4-year extended observation period in 4S; and 8-year observation period in LIPID) and have shown that relative risk reduction persists beyond the end of the formal double-blind phase.64,65
The LIPID trial initially compared pravastatin and placebo in over 9,000 patients with ASCVD over 6 years, following which, in 2016, the investigators published 16 years’ follow-up data.66 During extended follow-up, 85% of patients in the pravastatin group and 84% in the placebo group had continued statin therapy. The pravastatin group maintained a significantly lower risk for ASCVD, cardiovascular events and all-cause mortality.67
A meta-analysis of 58 clinical trials and 148,321 patients examined the reduction in ischaemic heart disease risk by duration of statin treatment. This study demonstrated that for every 1.0 mmol/l reduction in LDL cholesterol level, the ischaemic risk reduction was 11% in the first year and 36% in the sixth and subsequent years. Results of this meta-analysis suggested that longer durations of statin treatment are associated with greater reductions in ASCVD risk.68
New Frontiers in Lipid-lowering Therapies
Since it was discovered that statins could lower blood cholesterol and cardiovascular risk, multiple alternative strategies have struggled to replicate their effectiveness. New therapies have demonstrated significant promise at LDL cholesterol reduction and potential improvements in clinical outcome and are pertinent in patients who do not tolerate statin therapy; however, future cardiovascular risk remains significant.
Proprotein Convertase Subtilisin/Kexin Type 9 Inhibitors
PCSK9 has emerged as a therapeutic target to treat hypercholesterolaemia after observational registries reported nonsense mutations of the PCSK9 gene were associated with a substantial reduction in LDL cholesterol levels and incidence of coronary events.69
The FOURIER and Odyssey OUTCOMES evaluated evolocumab in patients with established cardiovascular disease and alirocumab in patients with recent ACS, respectively, while a large meta-analysis demonstrated a significant reduction in MI, ischaemic stroke and coronary revascularisation compared with placebo without a significant reduction of all-cause and cardiovascular death.70–72 The latest ESC guidelines emphasise the use of combination therapies, initially with ezetimibe and then PCSK9 inhibitors in patients with ACS.73
Inclisiran
Inclisiran is a chemically modified double-stranded small interfering RNA molecule administered subcutaneously with a prolonged effect against PCSK9 synthesis in hepatocytes.74,75 Two Phase III trials have shown significant LDL cholesterol reduction, while the ongoing ORION-4 trial will evaluate the impact of inclisiran in ~15,000 patients with established cardiovascular disease, including prior MI, on composite cardiovascular outcomes; patients experiencing an acute coronary event within 4 weeks of randomisation will be excluded.76,77
Bempedoic Acid
Bempedoic acid is an oral prodrug that is converted to its active thioester only in hepatocytes – the only cell to express the relevant acyl coenzyme A synthetase – inhibiting ATP citrate lyase, a key enzyme of the cholesterol-biosynthesis pathway.78 When combined with maximally tolerated statin therapy, bempedoic acid has led to an additional 15–20% reduction in LDL cholesterol and is now approved by NICE and the Food and Drug Administration in specific patient groups, although the impact on cardiovascular outcomes remains to be determined from the recently completed CLEAR Outcomes trial.79–82
Conclusion
Despite significant advancements in the initial treatment and secondary prevention of ACS, a considerable proportion of ACS survivors still experience subsequent cardiovascular events and mortality rates remain unacceptably high. Therefore, understanding the current evidence base to optimise lipid management strategies following an ACS event is crucial to improving cardiovascular outcomes in these patients.
Guidelines recommend obtaining a lipid profile soon after admission for ACS, followed by systematic evaluation of lipid-lowering therapy effectiveness. Statins, PCSK9 inhibitors and ezetimibe have demonstrated safety and efficacy in lowering LDL cholesterol levels and can be prescribed in ACS patients.
Early initiation of statin therapy, ideally within 24–48 hours of admission following ACS, is recommended. Combining ezetimibe with statin therapy provides further LDL cholesterol reduction and may offer greater benefits in reducing cardiovascular events than statin monotherapy.










