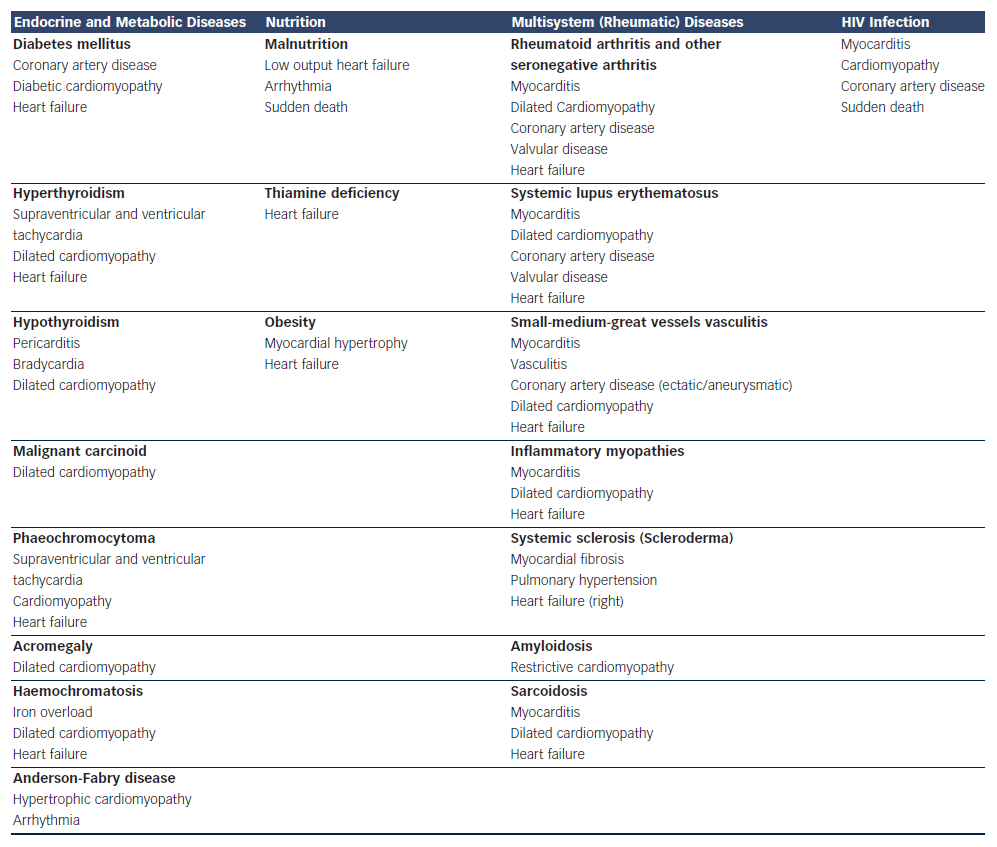
Systemic means ‘pertaining to or affecting the whole body’ as opposed to a localised condition. A systemic disease is one that affects a number of organs and tissues, or the body as a whole. Systemic diseases, according to WHO classification,1 and cardiac diseases that developed during their course, are listed in Table 1.
Thyroid diseases, pheochromocytoma and growth hormone excess may contribute to usually reversible dilated cardiomyopathy.2 Hereditary haemochromatosis is an inherited disorder of iron metabolism; if left untreated, leads to tissue iron overload, iron cardiomyopathy and heart failure. Cardiac amyloidosis is the result of amyloid deposition in the heart, formed from breakdown of normal or abnormal proteins that lead to increased heart stiffness, restrictive cardiomyopathy and heart failure. Finally, nutritional disturbances and metabolic diseases, such as Kwashiorkor, Beri-beri, obesity and diabetes mellitus may also lead to dilated cardiomyopathy and heart failure.3
Cardiovascular involvement is a common and underestimated problem in multisystem (rheumatic) diseases and may present with disease associated cardiac involvement at diagnosis or later in the course of the systemic disease. It usually has a silent or oligo-symptomatic cardiac presentation and includes different pathophysiological mechanisms such as, myocardial inflammation, infarction, diffuse, subendocardial vasculitis, valvular disease and different patterns of fibrosis; furthermore, acuity of heart involvement may be underestimated due to non-specific cardiac signs, and most of patients are female and unable to exercise due to arthritis or muscular discomfort/weakness or may have limited acoustic window due to increased breast size.4
Finally, HIV-infected patients have an increased risk of coronary artery disease (CAD) and myocardial inflammation frequently leading to heart failure. This is due to different factors including: conventional risk factors, HIV-specific processes driving inflammation, coagulation and endothelial dysfunction.5
Cardiovascular magnetic resonance (CMR) through its ability to reliably assess cardiac anatomy, function, inflammation, stress perfusion-fibrosis, aortic distensibility, and iron and fat deposition, constitutes an excellent tool for early diagnosis of cardiovascular involvement, risk stratification, treatment evaluation and long-term follow-up of patients with cardiovascular disease due to systemic diseases.
Cardiovascular Magnetic Resonance Applications of Special Interest for Cardiovascular Evaluation in Systemic Diseases
Measurement of Volumes – Ejection Fraction
CMR measures ventricular volumes and ejection fraction non-invasively and without contrast agent.6 Due to its high reproducibility, it is ideal for serial follow-up of ventricular volumes, mass and function; compared with echocardiography, which is an operator-dependent technique, with the limitations of acoustic window, CMR is operator-independent and has high reproducibility.7
The majority of CMR data regarding volumes and mass in systemic disease are referred to patients with metabolic diseases and mainly to diabetes mellitus. In patients with type 1 diabetes mellitus (T1DM), it was documented by CMR that in addition to traditional cardiovascular disease risk factors, elevated mean haemoglobin A (1c) and macroalbuminuria were significantly associated with alterations in left ventricular (LV) structure and function.8 In overweight and obese women, insulin resistance is associated with increased cardiac remodelling and reduced diastolic function, assessed by CMR.9 Finally, osteoprotegerin (OPG) was inversely associated with aortic distensibility, LV volumes and LV diastolic function assessed by CMR, while adipocytokine adiponectin (ADPN) was positively associated with myocardial glucose metabolism (MMRglu) by 18F-2-fluoro-2-deoxy-D-glucose positron emission tomography.10 In another study, CMR was used to assess the effect of rosiglitazone on cardiovascular performance and cardiac function in type 2 diabetes mellitus (T2DM) and proved that rosiglitazone increased peripheral oedema but had no pernicious effects on cardiovascular performance or cardiac function, with modest improvement in selected CMR measures.11 Although insulin and glucose indices are associated with abnormalities in cardiac structure, insulin resistance and worsening glycaemia are consistently and independently associated with left ventricular mass (LVM)/LV end-diastolic volume (LVEDV). These data implicate hyperglycaemia and insulin resistance in concentric LV remodelling.12
CMR also documented that few patients with HIV may have a marginally reduced right ventricular ejection fraction (RVEF) but normal right ventricular (RV) dimensions and mass.13
Finally, the progression to heart failure in rheumatoid arthritis (RA) may occur through reduced myocardial mass rather than hypertrophy, and both modifiable and non-modifiable factors may contribute to lower levels of left ventricular mass and volume.14
Myocardial Ischaemia
CMR can detect ischaemia by two different ways:
- Observation of wall motion abnormalities (abnormal wall motion and wall thickening) using the stress factor dobutamine. Compared with stress echocardiogram, stress CMR using dobutamine has better sensitivity (86 % versus 74 %) and specificity (86 % versus 70 %).15–17
- Observation of myocardial perfusion by the first-pass of a bolus of a T1-shortening contrast agent (first-pass gadolinium) injected into a peripheral vein.18,19 Data acquired during intravenous vasodilatorstress (most commonly adenosine) delineate the underperfused regions associated with myocardial ischaemia. The spatial resolution of CMR myocardial perfusion imaging of 2–3 mm is greatly superior to other imaging modalities, such as nuclear techniques, so that subendocardial ischaemia can be more reliably identified.20,21 Recent developments led to further improvements in spatial resolution to around 1 mm in the imaging plane.22–24 The interpretation of CMR myocardial perfusion studies in clinical practice have been validated against X-ray angiography, single-photon emission computed tomography (SPECT) and positron emission tomography (PET).25,26 Recently, the Clinical Evaluation of Magnetic Resonance Imaging in Coronary Heart Disease (CE-MARC) study (the largest, prospective, real-world evaluation of CMR) has established its high diagnostic accuracy in coronary heart disease and superiority over SPECT; therefore it should be adopted more widely than at present for the investigation of coronary heart disease.27
Stress perfusion CMR has already been used for the evaluation of diabetic patients. In T1DM, myocardial perfusion reserve index (MPRI) was independently associated with increased LV torsion.28 In another study, it was documented that young subjects with uncomplicated T1DM have impaired myocardial energetics, irrespective of the duration of diabetes and the impaired cardiac energetics status results from metabolic dysfunction rather than microvascular impairment.29
Finally in a study by our group, adenosine stress CMR, using MPRI evaluation, detected early perfusion changes in asymptomatic T1DM, missed by the usual non-invasive evaluation.30
Stress perfusion CMR has also been applied in multisystem (rheumatic diseases). A 44 % prevalence of abnormal stress myocardial perfusion by CMR in the absence of obstructive CAD was documented in systemic lupus erythematosus (SLE) patients with anginal symptoms. Compared with controls, reduced MPRI was observed in SLE patients and SLE presence was a significant predictor of an abnormal MPRI. These findings are consistent with the hypothesis that anginal chest pain (CP) in SLE patients without obstructive CAD is due to myocardial ischaemia potentially caused by microvascular coronary dysfunction.31 Stress myocardial perfusion abnormalities were frequent in RA patients without known cardiac disease. Abnormal CMR perfusion findings were associated with higher RA disease activity, suggesting a role for inflammation in the pathogenesis of myocardial involvement in RA.32 Subclinical myocardial involvement, as detected by stress perfusion CMR, was frequent in asymptomatic patients with systemic sclerosis (SSc).33,34 Finally, in sarcoidosis without cardiac symptoms and normal routine assessment, stress perfusion CMR and myocardial inflammation assessment detected early cardiac involvement that may in some cases necessitate immediate treatment.35
Myocardial Viability (Fibrosis Detection or Viability Study)
Late Gadolinium Enhanced Imaging
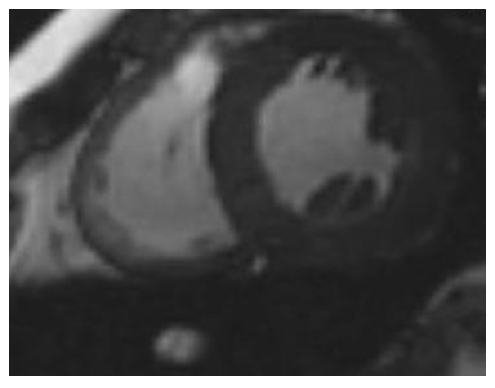
CMR is the most reliable imaging way to detect and quantify scar or fibrotic tissue, due to irreversible myocardial damage (viability study). Following acute ischaemic injury, the myocardial distribution volume of gadolinium is increased due to sarcolemmal rupture and abnormal wash-out kinetics. The preferred imaging time for scar detection is between 10 and 20 minutes after contrast agent administration, when the differences between scar, normal myocardium and blood pool are maximal. This method is referred in the literature as late gadolinium enhanced (LGE) CMR and is the gold standard for the in vivo assessment of myocardial scar (see Figure 1). CMR can detect infarction in as little as 1 cm3 of tissue, substantially less than other in vivo methods, such as echocardiography and nuclear techniques. Furthermore, CMR can detect subendocardial myocardial infarction, missed by SPECT/PET. The CMR extent of scar predicts the potential for functional recovery after revascularisation.36–38
Subendocardial and/or transmural LGE, following the distribution of coronary arteries, are indicative of CAD. However, not only the presence but also the LGE amount plays an important role in patients’ prognosis, because even a small area of LGE (<2 % of LV mass) was associated with a greater than seven-fold increase in risk for a major adverse cardiac event.39
CMR can characterise silent scar consistent with myocardial infarction (MI) in diabetic patients without clinical evidence of MI, and has strong association with major adverse cardiovascular events (MACE) and mortality hazards that is incremental to clinical, electrocardiogram (ECG) and LV function combined.40
LGE has already been described in vasculitis, myositis, SLE and RA; it may present different patterns including subendocardial or transmural lesions in the territory supplied by the occluded coronary artery, intra-myocardial or subepicardial not following the distribution of coronary arteries, mimicking the pattern of viral myocarditis and/or diffuse subendocardial pattern due to vasculitis.41
Finally, global subendocardial LGE was identified by CMR in severe cardiac amyloidosis.42
Iron Deposition Assessment
Patients with haemochromatosis are in great risk of iron overload. ‘T2-star’ (T2*) technique, assessed by CMR, is a non-invasive method for measuring liver and cardiac iron deposition (see Figure 2). A significant curvilinear, inverse correlation between iron concentration measured by biopsy and liver T2* was found. Myocardial iron deposition can be reproducibly quantified using T2*. This is the most significant variable for predicting a requirement for targeted treatment of myocardial iron overload and it cannot be replaced by serum ferritin, liver iron or any other measurement. Excellent T2* reproducibility between scanners produced by two different manufacturers supports the feasibility of widespread implementation of the technique. Myocardial T2* seems to be the most sensitive and easily reproducible index of myocardial iron deposition currently available.43
Therapeutic phlebotomy and iron chelation are the cornerstones of haemochromatosis therapy. The average survival is less than a year in untreated patients with severe cardiac impairment. However, if treated early and aggressively, the survival rate approaches that of the usual population with heart failure. CMR, using T2* measurements, can quantify myocardial iron overload and response to iron reduction therapy by serial imaging evaluation.43
Aortic Stiffness
Aortic distensibility and aortic pulse wave velocity (PWV) are two parameters closely related to the bio-elastic function of the aorta. Quantification of aortic distensibility and PWV by CMR has been shown to be accurate and reproducible and can identify early cardiovascular disease in asymptomatic patients. Gradient echo cine CMR has been applied to assess aortic distensibility and phase-contrast cine CMR to evaluate aortic PWV and to quantify aortic flow.44
In type 1 diabetes there are strong adverse effects of hypertension, chronic hyperglycaemia and macroalbuminuria on aortic distensibility.45 In another study, a combined CMR assessment of aortic PWV, aortic distensibility and heart function reveals abnormal PWV and distensibility in T2DM that is independent of blood pressure and correlates with diastolic LV function.46 Additionally, aortic elastic function is abnormal in obese subjects without other cardiovascular risk factors.47
Finally, juvenile idiopathic arthritis (JIA) is associated with increased aortic stiffness that might suggest subclinical atherosclerosis.48
Epicardial Fat
Fat deposits are often found around the heart. This fat can be separated into different compartments. Epicardial fat (EF) is the adipose tissue accumulated between the visceral pericardium and the myocardium, without a structure or fascia separating it from the myocardium and the epicardial vessels. EF has a variable distribution, being more prominent in the atrioventricular and interventricular grooves and right ventricular lateral wall.49 Adipocytes’ infiltration into the myocardium as well as triglyceride infiltration into myocytes may also occur.
The fat located on the outer surface of the fibrous pericardium differs from EF in their biochemical, molecular and vascular nutrition properties. It is nourished by the pericardiophrenic artery, a branch of the internal thoracic artery,49 while EF is nourished by the coronary arteries. The structure that delimitates these layers is the pericardium, seen on imaging tests as a thin layer around the heart, between 1.0 and 4.0 mm, of which visualisation is sometimes difficult.
CMR is considered as the gold standard for the assessment of total body fat and reference modality for the analysis of ventricular volumes and mass, thus making it a natural choice for the detection and quantification of EF.50 The total volume of EF can be estimated using the modified Simpson method, in which the epicardial tissue is contoured in each short axis at end of diastole. The interobserver reproducibility of EF volume measurement is superior to the EF thickness measurement (coefficient of variability of 5.9 % for the volumetric method and 13.6 % for EF thickness at the long axis); however, it is technically more difficult.50
In prepubertal and early pubertal obese children, EF is a significant marker of increased insulin resistance and associated cardiovascular risk.51 Additionally, subjects with type 1 diabetes have higher EF than non-diabetic subjects.52
Magnetic Resonance Angiography
Magnetic resonance angiography (MRA) is an imaging modality that comprises various techniques based on two concepts: methods relying on the natural flow effects, the time-of-flight and phase-contrast technique, either in two- or three-dimensional acquisition mode and the more recently developed contrast-enhanced (CE) MRA methods.
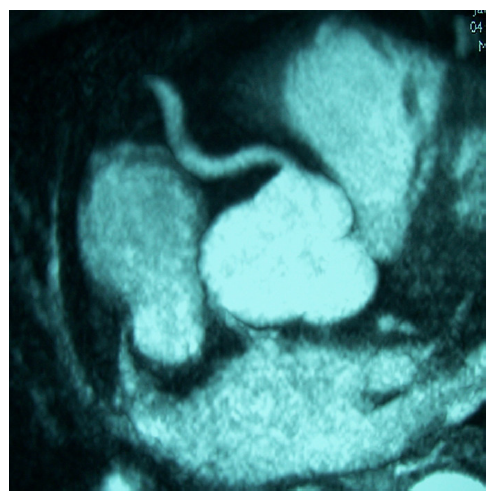
A study in diabetic patients, using whole-body magnetic resonance imaging (WB-MRI) and whole-body magnetic resonance angiography (WB-MRA), found a prevalence of 49 % for peripheral artery disease, 25 % for myocardial infarction, 28 % for cerebrovascular disease and 22 % for neuropathic foot. In all vessels, at least 50 % of pathologies were previously unknown. Additionally, myocardial infarction, chronic ischaemic cerebral lesions and atherosclerotic disease were significantly more common in diabetic than in controls.53 MRA has also been applied in the evaluation of great vessels in large vessels vasculitis, such as Takayasu disease (see Figure 3).54
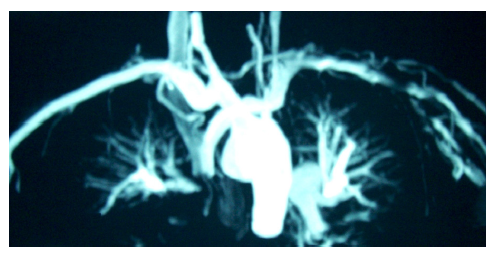
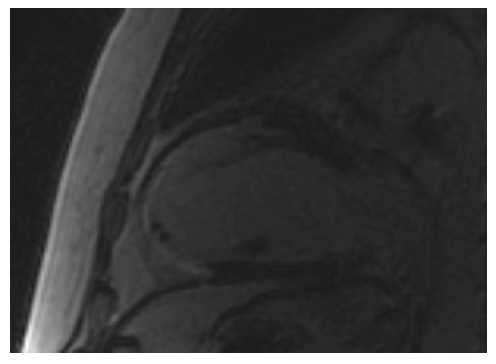
Coronary magnetic resonance angiography (CMRA) has already been applied in different systemic diseases. In diabetes mellitus, CMRA may detect early coronary artery changes.55 In patients with systemic antineutrophil cytoplasmic antibodies (ANCA)-related vasculitis, CMRA detected ectatic and/or aneurysmatic coronary arteries (see Figure 4).56 Additionally, in Kawasaki disease, CMRA is a useful tool for a radiation free serial coronary artery evaluation.57 Finally, in HIV patients, CMRA may facilitate the early detection of CAD.58
T1 Mapping
CMR has the capability to characterise myocardial tissue using T1 and T2 mapping techniques. Quantitative T1 imaging, in particular, can be used to calculate the myocardial extracellular volume fraction (ECV), a measure of microscopic myocardial remodelling that has been associated with underlying diffuse fibrosis
Diffuse myocardial fibrosis is an underlying contributor to early diabetic cardiomyopathy, as it was documented by the association between myocardial diastolic dysfunction, post-contrast T1 values and metabolic disturbance.59 Recently, it was documented that non-contrast T1 mapping had high diagnostic accuracy for detecting cardiac AL amyloidosis, correlated well with markers of systolic and diastolic dysfunction and was potentially more sensitive for detecting early disease than LGE imaging; therefore, elevated myocardial T1 may represent a direct marker of cardiac amyloid load.60 In patients with SLE without cardiac symptoms, T1 mapping may detect subclinical myocardial involvement.61
Myocardial Inflammation
CMR is the ideal technique for the evaluation of inflammatory processes involving the heart. Myocarditis often has a subclinical course, which cannot be easily detected with standard inflammatory indices evaluated in the blood (erythrocyte sedimentation rate [ESR], C-reactive protein [CRP], etc.) and can lead, under special conditions, to dilated cardiomyopathy.62 During its early stages it can also remain undetected by the commonly used imaging techniques (echocardiography or nuclear imaging techniques), because these techniques are unable to distinguish slight tissue structure changes (oedema, cell infiltration), which can occur without associated changes in LVEF, the most often detected parameter by echocardiography. Instead, CMR can perform tissue characterisation, which makes it extremely valuable in myocarditis.
According to current experience, in myocardial inflammation due to infectious causes (i.e. viral myocarditis), a decrease in LVEF was not evident during the course of the disease, while an increase in cardiac troponin was found in only 20 % of cases.20 Additionally, myocardial biopsy is an invasive procedure and according to the American College of Cardiology (ACC)/American Heart Association (AHA) guidelines should be kept only for patients with unexplained new-onset heart failure <2 weeks in duration associated with a normal-sized or dilated left ventricle in addition to haemodynamic compromise, and cannot be used for screening or follow-up tool.62 Furthermore its diagnostic value is limited due to a number of reasons (sampling error, variation in observer expertise, etc.).61
CMR contributes to the diagnosis of myocarditis using three types of images: T2-weighted (T2W), early T1- weighted (T1W) images taken at one minute and delayed enhanced images (LGE) taken 15 minutes after the injection of contrast agent.
T2W is an indicator of tissue water content, which is increased in inflammation or necrosis, such as during myocardial infarction or myocarditis. However, it is not possible to differentiate between necrosis and inflammation only by the use of T2W images. To enhance the detection of pathology on CMR, images after early and delayed gadolinium injection should be obtained. Higher levels of early myocardial enhancement after gadolinium administration are due to increased membrane permeability or capillary blood flow. Membrane permeability is a major contributor as inflammation damages cell membranes through both T-cell perforin and B-cell antibody/ complement-mediated processes. The third parameter, which should be also evaluated, is the presence of contrast agent deposition in the delayed images (LGE images) (see Figure 5). Myocardial necrosis in the acute phase appears to play a major role in LGE formation, but also severe oedema could increase the gadolinium distribution and cause delayed enhanced areas. A combined CMR approach using T2W, early and late gadolinium enhancement, has a sensitivity of 76 %, a specificity of 95.5 % and a diagnostic accuracy of 85 % for the detection of myocardial inflammation.62,63
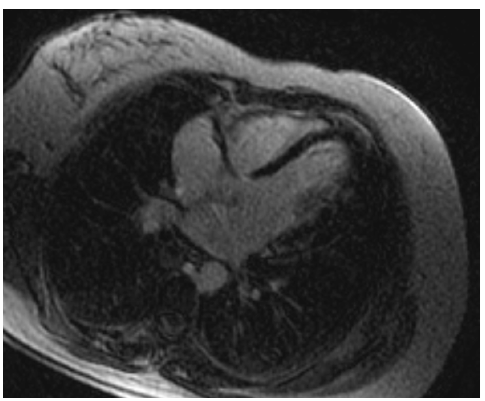
Evaluation of inflammation by CMR has already been applied in pheochromocytoma,64 diabetes mellitus,65 endocrine2 and rheumatic diseases,4 and allowed the detection of pathophysiology of cardiac lesions as well as cardiac disease acuity.
Limitations of Cardiac Magnetic Resonance
- Lack of availability;
- high cost;
- high expertise level needed for accurate diagnosis;
- awareness of referring physicians about the applications of the technique; and
- claustrophobia, metallic clips, pacemakers (unless CMR compatible), defibrillators.
Conclusions
CMR, using different sequences, can be of great value for the early and accurate assessment of cardiac involvement due to systemic diseases. CMR is a powerful technique that offers reliable and operator-independent assessment of cardiac anatomy, function, stress perfusion-fibrosis, myocardial-vessel inflammation, aortic distensibility, and iron and fat deposition; additionally it can identify disease acuity. These applications make the technique an excellent tool for heart-vessels assessment, risk stratification and treatment evaluation of patients with systemic diseases.