Lower Extremity Artery Disease: Definition, Epidemiology and Clinical Presentation
Lower extremity artery disease (LEAD), primarily an atherosclerosis -driven disease, causes varying degrees of disturbances in blood perfusion to the lower limb.1,2
The prevalence of LEAD in the general population has been reported to be 3–10%, although prevalence varies depending on the age of the population, reaching around 18% for those aged ≥65 years.2,3
In early stages, LEAD is usually asymptomatic, and the impaired limb circulation is only evident as an ankle–brachial index (ABI) ≤0.90. With progression of the disease, intermittent claudication (IC) may occur, characterised by muscle fatigue and lower extremity pain triggered by physical activity and directly relieved when resting.4 In the more severe critical limb-threatening ischaemia (CLTI), the patient experiences pain at rest and/or presents with ischaemic ulceration or gangrene of the foot.4
The mere presence of LEAD, either asymptomatic or symptomatic, is a significant predictor of increased risk of cardiovascular events (CVE) and cardiovascular mortality.5–7 Among individuals with IC, there is a low risk of progression to CLTI, with only 2% requiring lower extremity amputation within 10 years from diagnosis.8 Conversely, in the CLTI population, first-year rates of amputation in most studies are greater than 15–20% and 1-year mortality rates increase markedly from a few per cent in IC to 20–30% in CLTI.9,10
Compensatory development of new capillary networks (angiogenesis) and expansion of collateral arteries (arteriogenesis) are physiological responses to progressing limb ischaemia also encompassing pathological processes such as inflammation, apoptosis and vascular remodelling.11 In the constantly hypoperfused tissue in CLTI, chronic inflammation induces endothelial dysfunction and oxidative stress, with subsequent mitochondrial damage, generation of free radicals, muscle degeneration, connective tissue damage, fibrosis and eventually risk of gangrene.12–14 These events are possible targets for treatment and potential sources of diagnostic and prognostic biomarkers.
Methods
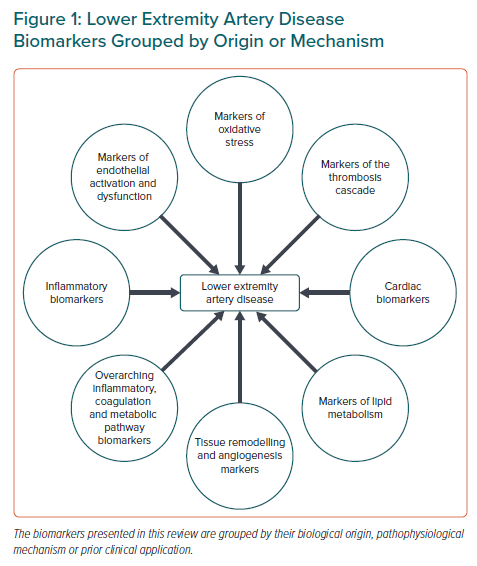
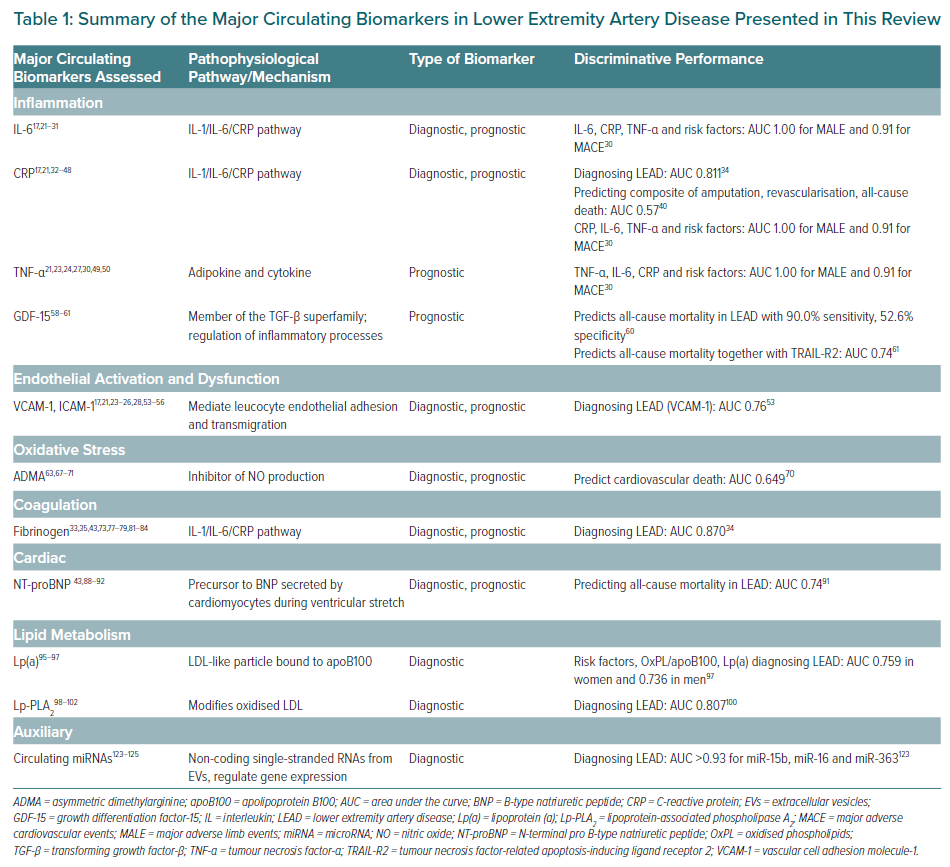
The aim of this review is to provide a comprehensive overview of the most promising established and emerging circulating biomarkers for LEAD and to discuss the usefulness of these biomarkers in screening, risk stratification and monitoring of therapeutic effect in line with personalised medicine. The biomarkers are grouped according to their mechanism in atherothrombotic disease, as shown in Figure 1. In addition, major biomarkers with data regarding their discriminative performances are summarised in Table 1.
The search terms used in this review were: ‘lower extremity artery disease’ or ‘peripheral artery disease’ or ‘intermittent claudication’ or ‘critical limb-threatening ischemia’ or ‘critical limb ischaemia’ and ‘biomarker’. In additional searches, the term ‘biomarker’ was changed to the names of specific biomarkers.
Biomarkers: Introduction and Definitions
The definition of a biomarker used in this review is the one presented by the Food and Drug Administration and the National Institutes of Health, specifically: “A defined characteristic that is measured as an indicator of normal biological processes, pathogenic processes or responses to an exposure or intervention.”15 The biomarker concept should be distinguished from clinical outcome assessments (i.e. evaluations of mental or physical status). To be of clinical value, a biomarker must be stable in the pre-analytical context, measurable without too much effort or cost and provide information on diagnosis and/or prognosis specific to the condition in question. In addition, a novel biomarker should add information incremental to the diagnostic tools and markers already used in clinical practice.
In this review, we focus on circulating biomarkers evaluated for diagnosis or assessment of prognosis in LEAD in combination with existing clinical biomarkers.
Circulating Biomarkers
As an adjunct to the clinical assessment tools, circulating biomarkers may contribute to the diagnostics of LEAD, prediction of future deterioration, including cardiovascular disease (CVD) progression in general, and evaluation of treatment effects. Despite demonstrated associations and biological relevance, most biomarkers are insufficient to reclassify individuals correctly above and beyond existing clinical and circulating biomarkers, such as ABI, which carries strong predictive power for CVD mortality.16 Moreover, due to large intraindividual variations in concentration, single-biomarker assessments may be of limited value.17 The biomarkers presented in this review are grouped by pathophysiological origin and mechanism (Figure 1).
Inflammatory Biomarkers
LEAD is primarily a consequence of progressive atherosclerosis, with or without atherothrombosis, in the lower extremity vasculature, with resulting ischaemia and subsequent tissue damage. Given the established contribution of low-grade chronic inflammation in all stages of atherosclerosis, it is conceivable that inflammatory processes, such as those reflecting endothelial cell activation, synthesis and secretion of proinflammatory factors, the expression of adhesion molecules for inflammatory cells and prothrombotic activity, have been targeted in the search for disease biomarkers. These early processes, including monocyte recruitment, are driven by the innate immune system, and inflammatory cells or tissue cells activated during inflammation have been associated with a poor prognosis in LEAD.18,19
Interleukin-6
Interleukin (IL)-6, central in atherosclerosis development and progression, is part of the IL-1β/IL-6/C-reactive protein (CRP) pathway. IL-6 signals through two different pathways, with the classical IL-6 signalling pathway being the main mediator of the acute phase reaction with subsequent expression of adhesion molecules and the proliferation and transformation of vascular smooth muscle cells into foam cells.20 The proinflammatory IL-6 trans-signalling pathway has not been analysed in LEAD.
In several case-control studies, IL-6 concentrations were higher in individuals with LEAD.17,21–23 This relationship was true also before and after treadmill exercise.24 In the Edinburgh Artery study of middle-aged men and women, IL-6 was associated with deterioration in ABI after 5 and 12 years follow-up independent of cardiovascular risk factors, baseline CVD and ABI.25 In line with the Edinburgh cohort, later studies have demonstrated that walking endurance in LEAD patients was inversely associated with high IL-6 concentrations.21,26,27 Moreover, persistently elevated IL-6 concentrations were associated with a more rapid functional decline compared with subjects with fluctuating or persistently low IL-6 concentrations.28 In addition, decreased size of the calf muscle as a surrogate marker of impaired leg function was associated with IL-6.29 In CLTI patients with diabetes, IL-6 was correlated with negative vascular outcome after endovascular revascularisation and was an independent predictor of in-stent restenosis 6 months after stenting in the femoropopliteal artery.30,31
C-Reactive Protein
CRP is an acute-phase reactant and product of classical IL-6 signalling. Several studies have shown that CRP is associated with the risk of CVE, although causality has not been demonstrated. Several case-control studies reported higher CRP concentrations in subjects with LEAD than in healthy controls.17,21,32–34 The Physicians’ Health study and the population-based prospective ARIC cohort study demonstrated that CRP independently predicted incident LEAD.35,36 In the Rotterdam cohort study, CRP predicted progression of atherosclerosis, including LEAD, independently of traditional cardiovascular risk factors and in the Edinburgh Artery study and a prospective study by Aboyans et al., the same was shown specifically for LEAD.25,37,38 However, not all studies did demonstrate an association between CRP and LEAD.39
In patients with symptomatic LEAD, CRP was associated with an increased risk of future atherothrombotic events both in the lower extremities and in other vascular beds.32,40–42 Combining high-sensitivity C-reactive protein (hsCRP) measurement with the assessment of ABI improved cardiovascular risk stratification in patients with LEAD.32,43 However, hsCRP alone has been shown to be inferior to ABI in the prediction of disease severity.44 These results were confirmed by a systematic meta-analysis of prospective studies investigating CRP as a predictor of CVE in LEAD patients.45 Moreover, several studies have shown an inverse association between CRP and ABI, severe clinical LEAD and functional deterioration.19,25,33,34,46,47 High CRP concentrations also correlated negatively with walking duration.21,48 In addition, high CRP was associated with an enhanced risk of postoperative vascular events and failure of endovascular revascularisation in LEAD patients.30
Tumour Necrosis Factor-α
Tumour necrosis factor (TNF)-α is a central mediator of inflammatory reactions inducing matrix metalloproteinase synthesis and contributing to atherosclerotic plaque instability. In case-control studies, TNF-α and circulating TNF-α receptor levels were higher in patients with LEAD than in healthy subjects.21,23,49 In a pre- and post-exercise analysis, TNF-α concentrations were still higher in subjects with LEAD, regardless of treadmill training.24 Moreover, TNF-α expression and circulating levels of TNF-α were inversely associated with walking duration.21,50 Conversely, in a study of patients with verified LEAD, TNF-α concentrations were significantly correlated with an angiographic score, but an association with treadmill performance could not be demonstrated.27 TNF-α concentrations were associated with increased vascular risk in CLTI patients with diabetes after endovascular intervention.30
Markers of Endothelial Activation and Dysfunction
Adhesion Molecules
Cell adhesion molecules (CAMs) are transmembrane glycoproteins that create binding sites for cell–cell and cell–extracellular matrix adhesion. CAMs, expressed on vascular endothelium and leucocytes subsequent to proinflammatory stimuli, mediate the tethering, rolling, adhesion to the endothelium and transmigration of recruited leucocytes into the subendothelial layer. In atherosclerosis, the three CAM families of importance are selectins, the immunoglobulin superfamily and integrins.
Selectins
Selectins are involved in inflammatory responses, such as atherosclerosis, and consist of three different types: E-selectin, expressed on endothelial cells; L-selectin, expressed on leucocytes; and P-selectin, expressed on platelets and endothelial cells.
Significantly higher concentrations of E-, L- and P-selectins have been reported in LEAD patients compared with controls.17,22,23 In addition, higher plasma P-selectin was associated with increased LEAD risk in MESA.51 However, the results regarding selectins in LEAD are not consistent.52
Intercellular Adhesion Molecule-1 and Vascular Cell Adhesion Molecule-1
Soluble intercellular adhesion molecule (sICAM-1), part of the immunoglobulin superfamily, is mainly expressed on endothelial cells and leucocytes, whereas soluble vascular cell adhesion molecule-1 (sVAM-1) is restricted to vascular endothelial cells, where it mediates leucocyte–endothelium adhesion and promotes signal transduction between adhered cells.
Several studies have demonstrated higher sICAM-1 and/or sVCAM-1 concentrations in LEAD patients compared with healthy controls, and these adhesion molecules have been proposed as suitable for the detection of LEAD.17,21,23,24,53 When investigating biomarker levels in relation to exercise, VCAM-1 and ICAM-1 concentrations were higher in LEAD patients both before and after treadmill exercise.24 ICAM-1, but not VCAM-1, was independently associated with an increased risk of progressing to symptomatic LEAD in the prospective Physician’s Health Study that included apparently healthy middle-aged men.54 These results in men were later reproduced in both women and men in the Edinburgh Artery Study and in women exclusively in the Women’s Health Study.25,55 In addition, higher sICAM-1 and sVCAM-1 concentrations were associated with reduced walking ability in some studies, whereas there was no association between sVCAM-1 and walking capacity in another study.21,26,28 However, sVCAM-1 was associated with reduced calf muscle area and strength in LEAD.29 Regarding cardiovascular risk, high sVCAM-1 concentrations predicted increased risk in individuals with LEAD and improved the prognostic value of ABI.56
Other Circulating Inflammatory Biomarkers
Neopterin, synthesised by activated macrophages upon interferon-γ stimulation, possesses pro-oxidant properties and is a marker of macrophage activity and inflammation in atherosclerosis. Neopterin concentrations have been demonstrated to be higher in asymptomatic and symptomatic LEAD patients than in healthy controls and to be negatively correlated with ABI.23,34,57 In addition, neopterin was found to be an effective predictor of LEAD.34
Growth differentiation factor (GDF)-15 is involved in the regulation of cell growth, repair and apoptosis. GDF-15 is constitutively expressed in the reproductive organs, although its expression can be swiftly induced in other cell types by proinflammatory cytokines, such as IL-1β and TNF-α. Several studies have demonstrated GDF-15 to be a biomarker of acute coronary syndrome and mortality in coronary artery disease (CAD).58 In addition, GDF-15 concentrations are stable over time in CAD patients, suggesting that it is a marker of chronic disease.58 In two cohorts with asymptomatic and symptomatic LEAD, including CLTI patients, strong correlations were demonstrated between circulating GDF-15 and future lower extremity amputation and all-cause mortality.59 Moreover, GDF-15 predicted these outcomes equally efficiently as the combination of nine traditional vascular risk factors.59 Other studies have confirmed that GDF-15 may be an effective predictor of all-cause mortality in LEAD patients.60,61 Thus, circulating GDF-15 could be of value in predicting which CLTI patients would benefit from intensified treatment and/or surgical intervention.
Markers of Oxidative Stress
Oxidative stress is one of the pathogenetic mechanisms underpinning atherosclerosis, with the production of reactive oxygen species (ROS) and reduction in nitric oxide (NO) being associated with endothelial dysfunction. In LEAD, dysfunctional mitochondria mediate ROS production in lower extremity muscles.62 The short ROS half-life prevents direct measurement, thus more stable molecular targets of ROS can function as indirect oxidative stress markers. Markers of oxidative stress, such as asymmetric dimethylarginine (ADMA), and 8-hydroxy-2-deoxyguanosine (8-OHdG), have been seen to be elevated in LEAD.63,64 Moreover, in a case-control study, men and women with LEAD had lower serum concentrations of NO metabolites and higher concentrations of the main producer of ROS, the NADPH oxidase 2-derived peptide (NOX2-dp) compared with controls.65 In fact, NOX2-dp exhibited a negative correlation with ABI.65 Levels of the oxidative stress marker 8-OHdG were also inversely correlated with markers of NO generation, indicating a connection between NO shortage and oxidative stress in LEAD.64 Furthermore, 8-OHdG levels were correlated negatively with walking capacity.64
Asymmetric Dimethylarginine
ADMA is the natural inhibitor of NO synthase, an enzyme catalysing NO production, by affecting the NO precursor arginine. Thus, ADMA is a potential marker of endothelial dysfunction, and several studies have demonstrated associations between elevated circulating ADMA concentrations, traditional cardiovascular risk factors and an increased risk of CVD.66 Accordingly, plasma ADMA concentrations were higher in LEAD patients than in controls in a case-control study.63 In another case-control study, ADMA concentrations did not differ between patients with CLTI, IC and healthy controls, whereas the ratio between arginine and ADMA was lower in CLTI patients than in IC patients and healthy controls.67 Consistent data from prospective cohorts of asymptomatic or symptomatic LEAD patients have shown that increased plasma ADMA concentrations predict future CVE and both cardiovascular and all-cause mortality.68–71 Taken together, the available data suggest that ADMA may be a promising biomarker above all for detecting morbidity and mortality in LEAD patients.
Homocysteine
Circulating homocysteine (Hcy) mediates endothelial dysfunction when elevated. Moreover, there is evidence that Hcy potentiates the production of ROS.72 Approximately 30% of individuals with LEAD had increased circulating Hcy concentrations, compared with 1% in the general population.8 In addition, high Hcy concentrations were associated with worse functional outcome in LEAD.26
Markers of the Coagulation Cascade
With endothelial dysfunction and subsequent inflammation and atherosclerosis, the local haemostatic balance is shifted towards a procoagulant state accompanied by resolving fibrinolysis, which is more distinct in LEAD than in CAD.73
Procoagulant Markers
In both the COMPASS and VOYAGER trials, studying prophylactic treatment with the combination of aspirin and a low dose of the oral anticoagulant rivaroxaban in individuals with LEAD, a reduction in adverse lower limb events, cardiovascular death, MI and stroke was seen, corroborating the central role for a prothrombotic state in the pathology and complications of LEAD.74–76
Plasminogen Activator Inhibitor-1
The serine protease inhibitor plasminogen activator inhibitor (PAI)-1 prevents fibrinolysis and heightens the hypercoagulable state by inhibiting tissue plasminogen activator (tPA). In case-control studies, PAI-1 concentrations were higher both at rest and after exercise in LEAD patients compared with healthy controls.17,77–79
Thrombin Activation
Circulating concentrations of thrombin fragment 1+2 (F1+2) and thrombin–antithrombin III complex (TAT) are specific and sensitive markers of thrombin generation and have been seen to be higher in LEAD patients compared with controls.80 In addition, CLTI patients have even higher TAT concentrations than IC patients.79 In a case-control study of CLTI patients scheduled for revascularisation, CLTI patients preoperatively exhibited a prothrombotic condition together with fibrinolysis mirrored by increased TAT and fibrinogen and enhanced tPA and D-dimer compared with controls.73 Immediately after reperfusion, F1+2 and TAT had further increased as a sign of thrombin generation, whereas fibrinogen concentrations were decreased.73 This prothrombotic and fibrinolytic state persisted throughout the first postoperative month. In another study, subjects with LEAD exhibited increases in TAT and thrombin formation after exercise.77
Platelet-Activating Factors
Tissue Factor and von Willebrand Factor
Tissue factor (TF) and von Willebrand factor (vWF) are part of the initial steps of the coagulation cascade, with the damaged integrity of the endothelium exposing TF to coagulation factors (F) VII and FVIIa and vWF promoting platelet adhesion.
Concentrations of both TF antigen and vWF were higher in individuals with LEAD compared with non-LEAD controls, and TF antigen concentrations were higher in CLTI than in other stages of LEAD.78,79 Moreover, subjects with LEAD exhibited a general platelet-activating state with elevated concentrations of platelet factor 4, sVCAM-1 and P-selectin.80
Fibrinogen
Fibrinogen is an acute-phase reactant regulated by IL-6 and a marker of inflammation. In addition, fibrinogen stimulates platelet aggregation and is converted to fibrin by thrombin. Fibrinogen concentrations have been reported to be higher in individuals with LEAD than in healthy controls.33,77–79 In some studies, circulating fibrinogen predicted LEAD, including IC.35,81–83 Moreover, fibrinogen concentrations increased with the severity of LEAD.79 High fibrinogen concentrations were also associated with the risk of fatal CVE and predicted mortality in LEAD.43,84 Fibrinogen was higher in CLTI patients undergoing infrainguinal bypass compared with controls.73 In the same study, fibrinogen levels decreased immediately after reperfusion, possibly mirroring augmented thrombin-mediated conversion into fibrin.73
Markers of Fibrinolysis
D-Dimer
D-dimer, a protein fragment arising from dissolving blood clots, is an indirect marker of fibrinolysis and is thus associated with the presence of venous and arterial thrombosis.
Circulating D-dimer concentrations were higher in subjects with LEAD than in healthy controls before and after treadmill exercise.17,77–79 Moreover, data are available regarding associations between increased D-dimer concentrations and both the presence and severity of LEAD.33,79 High D-dimer concentrations have been shown to be associated with poor calf muscle characteristics and inferior functional capacity.26,29,48 In addition, increased D-dimer concentrations in LEAD predicted CVE risk and mortality.43,85,86 In CLTI patients admitted for revascularisation, active fibrinolysis mirrored by enhanced D-dimer levels was seen before intervention and persisted in the first month after the intervention.73 Despite plentiful research demonstrating the association between D-dimer and LEAD, there are conflicting data. In the prospective Edinburgh Artery Study, elevated D-dimer was associated with LEAD progression, although the association was not independent of the IL-1/IL-6/CRP pathway, and it was demonstrated that individuals with concomitant increases in D-dimer and IL-6 experienced the largest deterioration.87 Moreover, in a prospective study of individuals with LEAD followed for a median of 3 years, baseline D-dimer concentrations were neither associated with the risk of progression of LEAD nor with incident CVE, except for an increased risk of MI.39
Tissue Plasminogen Activator
tPA is a protease present on vascular endothelial cells and is active in the conversion of plasminogen to plasmin, mediating the dissolution of blood clots. Circulating concentrations of tPA antigen were increased in LEAD compared with healthy controls, and higher in patients with more severe disease.77,79 Subjects with LEAD had higher tPA antigen plasma concentrations at rest and after treadmill exercise.77 CLTI patients scheduled for revascularisation exhibited a prothrombotic state with high TAT and active fibrinolysis mirrored by enhanced tPA and D-dimer levels.73
Cardiac Biomarkers
Increased concentrations of N-terminal pro B-type natriuretic peptide (NT-proBNP), a marker of cardiac failure and myocardial ischaemia, have been reported in subjects with LEAD and, together with copeptin, were associated with the incidence of LEAD during long-term follow-up. 88,89 In the ARIC prospective cohort study, NT-proBNP and high-sensitivity troponin T (hsTnT), a marker of acute MI, were found to be predictive of incident LEAD.90 Moreover, high NT-proBNP and hsTnT independently predicted increased all-cause mortality in LEAD.43 In addition, hsTnT, but not carotid intima–media thickness or ABI, was predictive of reduced survival rate in a prospective LEAD cohort.91 Conversely, NT-proBNP was found to independently predict all-cause mortality after a 5-year follow-up in symptomatic LEAD patients,92 consistent with what has previously been demonstrated in heart failure.93
Markers of Lipid Metabolism
Oxidised LDL (oxLDL) possesses proatherogenic properties and, compared with healthy controls, levels of total cholesterol, LDL, oxLDL and oxLDL antibodies were higher in subjects with LEAD.94 In addition, oxLDL levels were positively correlated with total cholesterol and LDL.
Lipoprotein(a)
Lipoprotein(a), or Lp(a), is an LDL-like particle bound to an apolipoprotein B100 protein. Epidemiological studies and Mendelian randomisation (MR) analyses have demonstrated the association between Lp(a) and atherosclerotic CVD. Lp(a) concentrations and a single nucleotide polymorphism in its encoding gene LPA were also associated with LEAD, indicating causality between Lp(a) and LEAD.95 Circulating Lp(a) was an independent predictor of LEAD.96,97 In addition, Lp(a) concentrations were positively correlated with disease severity, total cholesterol, LDL and apolipoprotein B.96
Lipoprotein-Associated Phospholipase A2
Lipoprotein-associated phospholipase A2 (Lp-PLA2) contributes to oxLDL modification, one of the earliest steps in the atherosclerosis process. Lp-PLA2 binds to LDL particles in the circulation and is expressed by macrophages in atherosclerotic lesions. Lp-PLA2 activity and mass were, either alone or together with CRP, predictors of LEAD risk in two population-based cohorts of middle-aged or elderly individuals.98,99 These results were reproduced in a prospective cohort study analysing the risk of future LEAD-related hospitalisation associated with high Lp-PLA2 levels in subjects free of LEAD at baseline.100 In a hospital-based Chinese cross-sectional study, Lp-PLA2 concentrations were associated with the prevalence of LEAD independent of inflammatory markers such as hsCRP, Hcy and fibrinogen.101 Conversely, in a population-based US multi-ethnic cohort study of 45–84 year olds, Lp-PLA2 activity and mass were not associated with an increased risk of incident LEAD.102 Thus, results are conflicting regarding Lp-PLA2 as a predictor of LEAD.
Adiponectin
Adiponectin is an adipokine active in glucose and fatty acid metabolism that enhances insulin sensitivity and has anti-inflammatory and antioxidative properties. Lower adiponectin concentrations have been reported in men than in women, although adiponectin concentrations are lower in women with than without metabolic syndrome.103 These sex differences in anti-inflammatory adiponectin may contribute, in part, to higher CVD risk in men and women with metabolic syndrome. In relation to LEAD, adiponectin concentrations were lower in women who developed LEAD than in those that did not.104 In line with this finding in women, high adiponectin concentrations were associated with a decreased risk of developing symptomatic LEAD in men.105 Moreover, in men with symptomatic LEAD, but not in their female counterparts, low adiponectin concentrations were associated with a higher risk of non-fatal CVE.106
Tissue Remodelling and Angiogenesis Markers
Matrix Metalloproteinases
Matrix metalloproteinases (MMPs) are proteases with the ability to degrade extracellular matrix. MMPs are secreted by inflammatory cells and are active in vascular remodelling during the development and progression of atherosclerosis, including plaque rupture. MMP2 and MMP9 are also involved in the activation and regulation of platelet aggregation. In LEAD, concentrations of MMP2 and MMP9, and to some extent MMP3, were increased compared with healthy controls, and the progression and severity of LEAD have been associated with high concentrations of MMP2 and MMP9. 17,22,23,107
Galectin-3: Marker of Fibrosis and Calcification
Galectin-3 is induced by oxidative stress and is involved in inflammation, angiogenesis and fibrosis via mediation of cell–cell and cell–matrix interactions. Galectin-3 is also involved in macrophage maturation and has been associated with atherosclerotic CVD and LEAD.108 Levels of galectin-3 predicted incident LEAD and were higher in subjects with pathological ABI than in individuals without LEAD.36,109 Moreover, in LEAD patients, high galactin-3 concentrations were associated with an increased risk of cardiovascular mortality.108
Markers of Angiogenesis
Because angiogenesis is a physiological response to tissue ischaemia, circulating angiogenic factors may be relevant surrogates for disease severity via the increased production of angiogenic mitogens such as vascular endothelial growth factor (VEGF)-A and angiopoietin-2.
Vascular Endothelial Growth Factor-A
Increased circulating VGEF-A concentrations mirror pronounced angiogenesis. VGEF-A concentrations were higher in LEAD patients than in non-LEAD controls, and concentrations increased with the severity of the disease.110–112 Contrary to these findings, VGEF-A concentrations were lower in LEAD cases in a case-control study using controls with other cardiovascular risk factors and comorbidities other than LEAD.49 This finding highlights the difficulties with biomarkers mirroring processes active in systemic diseases. In relation to exercise-dependent changes in symptomatic LEAD, VGEF-A increased after home-based or supervised exercise.113,114
Angiopoietin-2 and the Tie2 Receptor
Together with VGEF-A, angiopoietin-2 stimulates neovascularisation. The cognate angiopoietin-2 receptor is the Tie2 receptor expressed mainly on the vascular endothelium.
In LEAD patients, circulating angiopoietin-2 and the soluble Tie2 receptor were increased compared with healthy controls.111 Moreover, no difference was seen in plasma angiopoietin-2 concentrations between IC and CLTI patients, whereas concentrations of the soluble Tie2 receptor were higher in CLTI than IC patients.111 In a prospective cohort of symptomatic LEAD, increased angiopoietin-2 was found to be independently associated with an enhanced risk of major adverse CVE and all-cause mortality.115
Overarching Inflammatory, Coagulation and Metabolic Pathway Biomarkers
Extracellular Vesicles
Circulating extracellular vesicles (EVs), including exosomes, microparticles and apoptotic bodies, are secreted by different types of cells upon stimulation, and carry nucleic acids, proteins, lipids and metabolites from the host cell, thereby mediating vascular homeostasis and intercellular communication. The concentrations and types of circulating EVs in the blood and the effects mediated by EVs differ depending on the stimulus causing their secretion. In LEAD, platelet-derived EVs are by far the most common type, followed by EVs from endothelial cells, erythrocytes and leucocytes.116 Case-control studies have demonstrated higher levels of platelet-derived EVs in individuals with LEAD than in healthy controls, although one study did not show any difference between the groups.116–118 In addition, levels of platelet-derived EVs were correlated with LEAD severity.119 Circulating levels of endothelial cell-derived EVs, especially when containing proinflammatory monomeric CRP, were higher in LEAD.120
Circulating MicroRNAs
MicroRNAs (miRNAs) are small, non-coding and single-stranded RNAs originating from EVs.121 MiRNAs are able to control gene expression at the post-transcriptional level, thereby inhibiting protein synthesis. MiRNAs are highly stable and expressed in a disease-specific manner, and therefore suitable as diagnostic biomarkers for CVD.122 A small case-control study performing transcriptomics on peripheral blood cells identified a group of miRNAs (miR-16, miR-363 and miR-15b) that had previously been associated with vascular pathophysiology as predictors of LEAD with outstanding diagnostic accuracy.123 In a larger case-control study, circulating (serum) concentrations of miRNAs (miR-130a, miR-27b, miR-210) were associated with LEAD.124 Moreover, in an all-male aortic aneurysm case-control study, four circulating miRNAs (let-7e, miR-15a, miR-196b and miR-411) were found to be associated both with aortic aneurysm and LEAD.125
Perspectives
Neither diagnostic nor prognostic circulating biomarkers for LEAD are used clinically today. When suspicion is raised, a diagnosis of LEAD with ABI is cheap and easy with a trained operator. In primary care, where sometimes both equipment and skills to measure ABI are lacking, circulating biomarkers would add value to screening for LEAD. Notwithstanding, disease-specific biomarkers predictive of incident LEAD are challenging to find because the organ-specific features of LEAD, primarily hypoperfusion with subsequent ischaemic tissue damage, are not present until later stages of the disease. Conversely, biomarkers indicating general atherosclerosis could function as a first-line screening marker for LEAD accompanied by ABI when appropriate. Later in the course of the disease, and certainly in symptomatic patients, organ-specific prognostic biomarkers may be of value together with imaging to stratify risk and evaluate the benefit of preventive medical treatment and/or interventional procedures. In addition, the association between LEAD and increased cardiovascular risk defines an important role for assessment of LEAD in personalised medicine5. Today, preventive medication for patients with LEAD is limited to drugs targeting traditional cardiovascular risk factors. In the development of novel, specific therapeutic targets for patients with LEAD, biomarkers representing pathways discussed in this review may be used to identify individuals suitable for treatment and to monitor the treatment effects of pharmacotherapies.
The traditional deductive approach in biomarker research has been challenged in recent years by inductive strategies using unbiased, large-scale, high-throughput plasma proteomic profiling. Such analyses demand large cohorts with enough statistical power to demonstrate associations and extensive bioinformatics to process large amounts of data. Conversely, new mechanisms and possible molecular targets can be discovered in corners previously not scrutinised.
Recent developments also highlight the future need for LEAD biomarkers. Using MR analysis and randomised controlled trials, the causal association between procoagulant factors and LEAD has been strengthened, and followed by the introduction of low-dose anticoagulant drugs in addition to antiplatelet therapy.126,75,76,2 However, biomarkers guiding the selection of patients for treatment and monitoring treatment effects are still lacking. The association between LEAD and inflammatory markers from the IL-1β/IL-6/CRP pathway displays consistent results in epidemiological studies and, in a recent randomised controlled trial, inhibition of the IL-1β/IL-6/CRP pathway with canakinumab dampened LEAD progression.127 In addition, positive associations between Lp(a) and LEAD in cohort and case-control studies, together with a demonstrated causal association between apolipoprotein B and LEAD in MR analyses, point to lipids as potential causal targets.128 With this is mind, the need for biomarkers in selecting patients for targeted treatment and the identification of individuals at risk of future hospitalisation for LEAD is pivotal. In such a setting, Lp-PLA2 holds great promise.100
Conclusion
The pathophysiology underpinning LEAD is multifactorial and, together with the effective diagnostic and predictive tools already at hand, will likely require a multiple-biomarker approach to provide incremental predictive value. Moreover, the impact of each causal pathway differs in individual patients, demanding biomarkers to evaluate the magnitude of the respective pathway in risk stratification assessments and to guide clinicians in which patients to treat and how to treat them. Many of the biomarkers presented in this review are associated with several inflammatory and/or atherosclerosis-related conditions, complicating interpretation. In this setting, the relatively new field of disease-specific circulating miRNAs as diagnostic and prognostic biomarkers is promising as analyses become cheaper and easier to use.122
Due to the late onset of symptoms and lack of diagnostic circulating biomarkers, LEAD patients are often diagnosed in late stages of the disease and, when diagnosed, biomarkers to guide the selection of patients for treatment and the monitoring of treatment effects are missing. However, the field is expanding, with many promising biomarkers continuously being investigated, together with potential targets for pharmacological treatment.