While the WHO has declared that COVID-19 is no longer a global health emergency, it has also emphasised that the disease remains a global threat.1 A number of risk factors are known to be associated with progression to severe disease, including cardiovascular disease (CVD) and related risk factors such as hypertension, diabetes and obesity.2,3 Accordingly, special guidance for management has been issued for patients with cardiovascular risk factors and COVID-19.4 The impact of CVD on COVID-19 is augmented by CVD being the most common cause of death globally, with more than 17 million deaths annually, accounting for more than 30% of all deaths worldwide.5,6 In Europe alone, more than 4 million people die each year from CVD.5,6
In addition, multimorbidity – in particular, cardiometabolic multimorbidity – along with polypharmacy that is common in the elderly, have been associated with a higher risk of progression to severe COVID-19.7–10 Clinicians thus require guidance for the use of medications in older adults with COVID-19, multimorbidity and polypharmacy.11
Like other RNA viruses, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) continually mutates, and variants continue to be a source of concern.12 While intense efforts were dedicated to use of vaccines to protect against COVID-19, increasing focus is being placed on antiviral therapies to treat COVID-19, given increased knowledge about the structure of the virus and its biology.13 Early antiviral use is recommended in patients who are at increased risk for progressing to severe COVID-19 and hospital admission.14 Among new antiviral therapies, the combination of nirmatrelvir/ritonavir (Paxlovid) has been approved for clinical use by the Food and Drug Administration and European Medicines Agency.
Patients with CVD are among those in whom early antiviral therapy should be warranted. Given that patients with CVD are often on polypharmacy, when treating COVID-19 with nirmatrelvir/ritonavir, physicians need to be aware of the potential drug–drug interactions (DDI) with this combination. This is relevant because not all prescriptions are appropriate in COVID-19 patients on polypharmacy.15 Commonly used drugs for CVD include antiplatelet agents, anticoagulants, statins, antiarrhythmics, antianginal agents, antihypertensives and anti-inflammatories. Since the pharmacokinetic booster ritonavir is an inhibitor of cytochrome P450 (CYP) 3A4, there is the potential for interactions with many drugs.16 This review summarises the clinical pharmacology data of nirmatrelvir/ritonavir and provides details on potential DDIs, with a focus on daily practice in patients with CVD.
Nirmatrelvir/Ritonavir
Nirmatrelvir is a SARS-CoV-2 main protease (Mpro) inhibitor with potent pan-human coronavirus activity.17 Mpro is an appealing target because it is essential in the viral replication cycle and has a low probability of off-target activity given the lack of human analogues.18,19 Nirmatrelvir is primarily metabolised by CYP3A4/5 and ritonavir is used as a pharmacokinetic enhancer or booster because of its potent and irreversible inhibition of the same cytochromes, allowing for less frequent dosing of nirmatrelvir.20 The combination has been shown to be highly effective in reducing the risk of severe COVID-19 and mortality.21
Pharmacokinetic and Pharmacodynamic Profile of Nirmatrelvir/Ritonavir
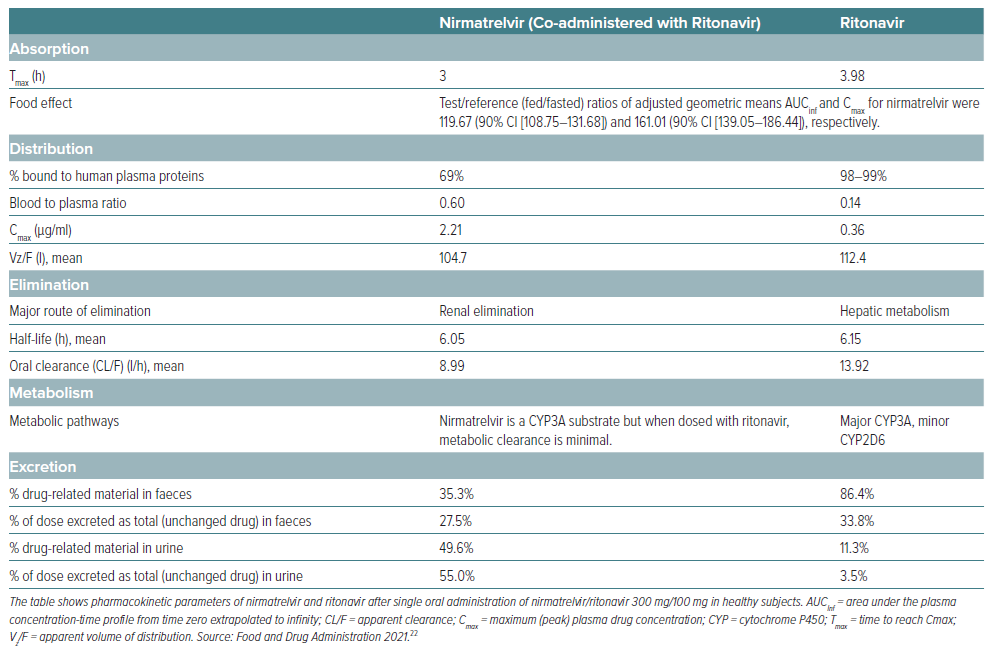
The main pharmacokinetic parameters are reported in Tables 1 and 2.22,23 In a Phase I study, the exposure and half-life of nirmatrelvir were considerably increased by ritonavir, and allowed selection of nirmatrelvir/ritonavir 300/100 mg twice daily for Phase II/III trials, which achieved concentrations continuously above those needed for 90% inhibition of viral replication in vitro.24 Following a single oral administration of nirmatrelvir/ritonavir 300/100 mg, the geometric mean Cmax of nirmatrelvir and ritonavir were 2.21 μg/ml and 0.36 μg/ml, respectively. The AUCinf of nirmatrelvir and ritonavir were 23.01 μg*h/ml and 3.60 μg*h/ml, respectively.25 The median Tmax were 3.0 and 3.98 hours for nirmatrelvir and ritonavir, respectively. The arithmetic mean terminal elimination half-life were 6.05 and 6.15 hours. As mentioned, ritonavir is an inhibitor of CYP3A4/5 and increases plasma concentrations of nirmatrelvir and other drugs that are primarily metabolised by CYP3A4/5. Despite being co-administered with ritonavir as a pharmacokinetic enhancer, there is potential for strong inhibitors and inducers to alter the pharmacokinetics of nirmatrelvir.25 Moreover, given the near irreversible inhibition of CYP3A4/5 by ritonavir there is enhancement of pharmacokinetic parameters of CYP3A substrate drugs that leads to their increased bioavailability.20 Nirmatrelvir/ritonavir can be taken with or without food.25
The pharmacokinetics of nirmatrelvir/ritonavir have been studied in patients with renal impairment and in those with end-stage renal disease.26,27 While no dose adjustments are needed for patients with mild renal impairment (estimated glomerular filtration rate [eGFR] ≥60 to <90 ml/min 1.73 m2), in patients with moderate renal impairment (eGFR ≥30 to <60 ml/min/1.73 m2), the dose should be reduced to nirmatrelvir/ritonavir 150/100 mg every 12 hours for 5 days to avoid over-exposure.25 The combination should not be used in patients with severe renal impairment (eGFR <30 ml/min/1.73 m2), including those with end-stage renal disease on haemodialysis.
There are limited data one the use of nirmatrelvir/ritonavir during pregnancy. Animal data with nirmatrelvir have shown developmental toxicity in rabbits (lower foetal body weights), but not in rats. A large number of women exposed to ritonavir during pregnancy indicate no increase in the rate of congenital abnormalities compared with rates observed in population-based birth defect surveillance systems.25 Animal data with ritonavir have shown reproductive toxicity.25 At present, nirmatrelvir/ritonavir is not recommended during pregnancy or in women of childbearing potential not using contraception unless the clinical condition requires treatment with the combination.25
Registrational Trial
Hammond et al. performed a Phase II–III double-blind study in which 2,246 symptomatic, unvaccinated, non-hospitalised adults at high risk for progression to severe COVID-19 were randomised to receive nirmatrelvir/ritonavir 300/100 mg twice daily or placebo for 5 days.21 The enrolment period was from 16 July 2021 to 9 December 2021. In the final analysis of 1,379 patients in the modified intention-to-treat population, there was a difference of −5.81 percentage points (95% CI [−7.78, −3.84]; p<0.001; relative risk reduction,88.9%) for COVID-19–related hospitalisation or death by day 28. Thus, compared with placebo, nirmatrelvir/ritonavir reduces progression to severe COVID-19 by 89% in unvaccinated, high-risk symptomatic patients. There were 13 deaths, all of which were in the placebo group. Moreover, viral load was lower with nirmatrelvir/ritonavir versus placebo at day 5 of treatment, with a similar incidence of adverse events between groups.21
Meta-analyses
Souza et al. carried out a meta-analysis of 16 observational studies on the efficacy of nirmatrelvir/ritonavir.28 Compared with standard treatment without antivirals, nirmatrelvir/ritonavir reduced the risk of death by 59% (OR = 0.41) and the risk of hospital admission by 53% (OR = 0.47). For a composite outcome of hospitalisation and/or mortality, nirmatrelvir/ritonavir reduced the risk by 56% (OR = 0.44). In a subgroup of patients aged <60 years, it appears there was no difference between treatment with nirmatrelvir/ritonavir compared with standard treatment (OR 0.48; 95% CI [0.09–2.50]); treating patients aged >60 years with nirmatrelvir/ritonavir suggested greater protection against the risk of death (OR 0.47; 95% CI [0.40–0.55]). On the other hand, nirmatrelvir/ritonavir reduced the risk of hospitalisation both those in aged <60 years (OR 0.45; 95% Cl [0.25–0.82]) and those aged >60 years (OR 0.30; 95% CI [0.13–0.70]), without a significant difference between the two groups
Chen et al. carried out a network meta-analysis of randomised controlled trials of oral small molecule drugs (azvudine, molnupiravir, nirmatrelvir/ritonavir, VV116 and placebo) that included nine trials on over 30,000 patients with COVID-19.29 The analysis found that nirmatrelvir/ritonavir significantly reduced both mortality (OR = 0.11) and hospitalisation (OR = 0.06) in patients with COVID-19, and was the drug that had the highest probability of being the best management strategy in patients with COVID-19.
Potential for Drug–drug Interactions with Nirmatrelvir/ritonavir
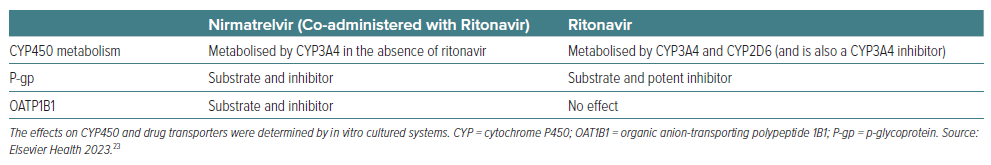
As mentioned, ritonavir has been used as a pharmacokinetic booster of other protease inhibitor antivirals predominantly because of its potent inhibition of CYP3A4.16 Given this, nirmatrelvir/ritonavir has a high potential to cause harm from DDIs with other drugs metabolised through this pathway.25,30 Cox et al. observed that co-administration of strong CYP3A4 inhibitor with a strong CYP3A inhibitor such as ritonavir was associated with small increases in plasma nirmatrelvir; co-administration of a strong inducer decreased systemic nirmatrelvir and ritonavir exposures.31 Additionally, in vitro studies showed that ritonavir also inhibits CYP2D6, which may determine an increase in the exposure of drugs metabolised by this cytochrome.23
Anticoagulants
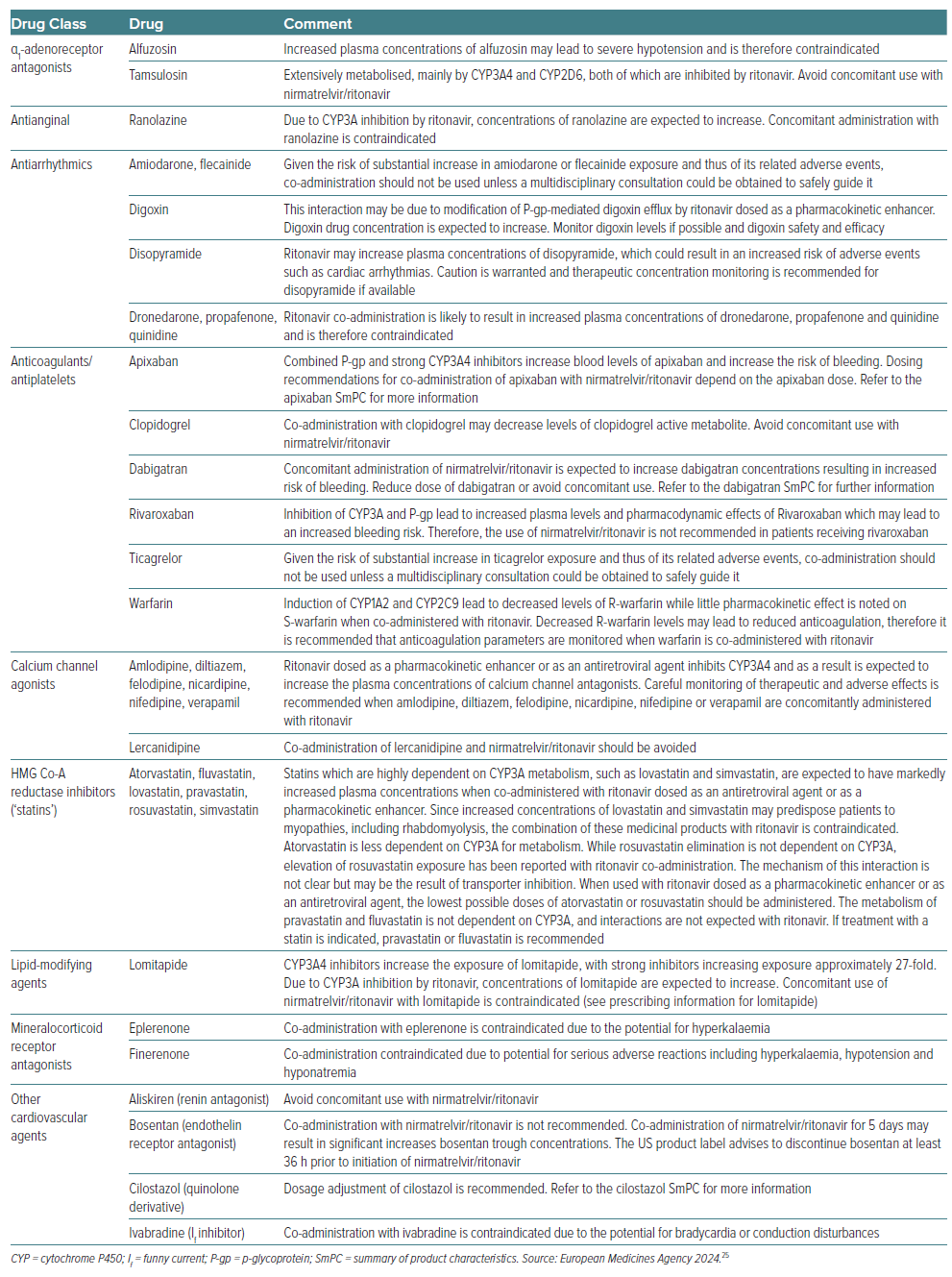
Exposure of P-glycoprotein (P-gp) substrates, such as direct oral anticoagulants (DOACs), may be increased by ritonavir.32 In healthy volunteers it has been noted that nirmatrelvir/ritonavir increased systemic exposure of dabigatran.33 In particular, nirmatrelvir/ritonavir increases the area under the concentration-time curve (AUC) and maximum (peak) plasma drug concentration (Cmax) of dabigatran by 94% and 133%, and by 153% (AUC) and 53% (Cmax) for rivaroxaban.25 Similar effects can be predicted for apixaban and edoxaban given that they are substrates of CYP3A4, and thus an interaction can be expected as for other DOACs.34
According to the Summary of Product Characteristics, monitoring of anticoagulation parameters is recommended during co-administration with warfarin.25 Muse et al. examined internal normalized ratio (INR) levels in a series of 29 patients treated with nirmatrelvir/ritonavir.35 While slight small changes in INR trends were observed, it was believed that these were related to the acute viral infection and not to treatment with nirmatrelvir/ritonavir. Notwithstanding, none of the patients had a severe infection requiring hospitalisation and thus INR changes due to an effect of the drug could not be ruled out.
Antiplatelet Agents
Among antiplatelet agents, ritonavir can theoretically increase aspirin metabolism, but no increase of clinical adverse events has been reported.36 Similarly, CYP3A4 and CYP2B6 are responsible for the bioactivation of prasugrel, a prodrug. Although there is a twofold decrease in the maximum concentration (Cmax) of prasugrel in patients on ritonavir, its antiplatelet activity does not seem to be affected.37 Thus, the co-administration of aspirin and/or prasugrel with nirmatrelvir/ritonavir should be considered safe.
Conversely, ritonavir decreases the production of the active metabolite of clopidogrel, reducing its platelet inhibition by 20%.37 Ticagrelor is a CYP3A4 substrate, and co-administration of nirmatrelvir/ritonavir is associated with an increased risk of bleeding.38 Thus, ticagrelor or clopidogrel should be replaced by prasugrel, or, alternatively, prescribing nirmatrelvir/ritonavir should be avoided and alternative COVID-19 therapies considered.
Antianginal Agents
With the exception of ranolazine, clinical effects and safety of antianginal agents (i.e. β-blockers and nitrates) are not significantly affected by nirmatrelvir/ritonavir. Ranolazine is a CYP3A4 and P-gp substrate.39 Its plasma concentration is exponentially increased in the presence of CYP450 inhibitors, thereby increasing the risk of QT prolongation and torsades de pointes. Therefore, co-administration of nirmatrelvir/ritonavir is contraindicated.22
Antiarrhythmic Drugs
Antiarrhythmic drugs (amiodarone, dronedarone, flecainide, propafenone and quinidine) are metabolised by CYP3A4 and co-administration with ritonavir is expected to significantly increase their exposure.25 For amiodarone and flecainide, co-administration should not be used unless a multidisciplinary consultation is obtained to safely guide it.25 Caution is warranted and monitoring of therapeutic concentration is recommended for disopyramide if available.25 Administration of dronedarone, propafenone and quinidine is contraindicated. Digoxin is expected to interfere with nirmatrelvir/ritonavir via direct inhibition of P-gp, and its administration can be managed by monitoring digoxin levels.25
Drugs for Heart Failure
Regarding heart failure drugs, angiotensin-converting enzyme inhibitors, ‘sartans’, spironolactone, sodium-glucose cotransporter 2 inhibitors and diuretics do not show significant interactions with nirmatrelvir/ritonavir. Thus, their continuation is allowed and safe with close blood pressure monitoring. A weak inhibition of the hepatic uptake transporter organic anion-transporting polypeptide 1B1 (OATP1B1) by nirmatrelvir/ritonavir may increase the concentration of both valsartan and the active metabolite of sacubitril, warranting close blood pressure monitoring and possibly dose reduction or temporary withdrawal of sacubitril/valsartan while on nirmatrelvir/ritonavir.40
Among mineralocorticoid receptor antagonists used to treat heart failure, eplerenone is primarily eliminated by CYP3A4,41 and concurrent nirmatrelvir/ritonavir administration can significantly increase the risk of hyperkalaemia and therefore is contraindicated.
Finally, ivabradine is a CYP3A4 substrate. Co-administration with nirmatrelvir/ritonavir may cause significant bradycardia and therefore co-administration with ivabradine is contraindicated.25
Antihypertensive Agents
Among antihypertensive agents, amlodipine, nifedipine and felodipine are metabolised by CYP3A4. Thus, the significant increase of blood levels may require dose adjustment and close monitoring of adverse effects, which may warrant temporary discontinuation of treatment while on ritonavir.22 Similarly, diltiazem and verapamil are metabolised by CYP3A4 and CYP2D6. Careful monitoring for adverse effects such as bradycardia, dizziness and hypotension, which may warrant dose adjustment or temporary discontinuation, is recommended when administered with nirmatrelvir/ritonavir.22
Finally, α-blockers, such as doxazosin and terazosin, are also metabolised by CYP3A4, and co-administration with nirmatrelvir/ritonavir can increase plasma concentration of these agents causing hypotension.42 Alfuzosin is contraindicated because of a risk of profound hypotension.22
Statins
The HMG-CoA reductase inhibitors simvastatin and lovastatin are highly dependent on CYP3A metabolism and are expected to have markedly increased plasma concentrations when co-administered with ritonavir.43 Thus, the combination of these drugs with ritonavir is contraindicated since they predispose patients to myopathies and rhabdomyolysis. Metabolism of atorvastatin is less dependent on CYP3A4, while rosuvastatin is independent from this pathway.43 However, elevation of rosuvastatin and atorvastatin plasma levels have been reported with ritonavir, potentially due to the inhibition of P-gp and OATP1B1.44 Thus, when used with ritonavir, the lowest possible doses of atorvastatin or rosuvastatin should be used.25
In summary, many classes of drugs can interfere with nirmatrelvir/ritonavir with a clinically relevant effect, and the potential entity of DDIs is large. In fact, it has been estimated that approximately one-third of the US population would be at risk for a major or contraindicated DDI should they receive a ritonavir-containing regimen, with the risk increasing significantly among individuals aged ≥60 years and with comorbidities, such as serious heart conditions, chronic kidney disease, diabetes and HIV.45 A Danish study reported that among drugs likely to interact with nirmatrelvir/ritonavir, anticoagulants that are contraindicated during nirmatrelvir/ritonavir treatment were being used by 20% of the population aged ≥65 years and 30% of those aged ≥80 years.46
It is essential not only that clinicians are aware of drugs that are contraindicated with nirmatrelvir/ritonavir, but also recognise which drugs can be safely co-administered.
Case Reports of Drug–drug Interactions with Nirmatrelvir/Ritonavir
Several case reports have documented diverse DDIs with nirmatrelvir/ritonavir and several drugs. Rauser et al. published the case of a patient experiencing a DDI between nirmatrelvir/ritonavir and nifedipine that gave rise to oedema, oliguria and acute kidney injury.47 The 79-year-old woman with multiple comorbidities (moderate-to-severe chronic kidney disease, type 2 diabetes, hypertension and congestive heart failure) on polypharmacy presented with cough and a positive test for SARS-CoV-2. Among her medications, colchicine and rosuvastatin were temporarily discontinued upon initiation of nirmatrelvir/ritonavir. However, the patient returned 3 days later with peripheral oedema and decreased urine output. Suspecting a DDI, nirmatrelvir/ritonavir and nifedipine were discontinued after which the oedema and renal function normalised within 2 days.47
Haque et al. reported on an elderly woman who presented with acute onset of generalised weakness, lethargy and altered mental state.48 Investigations led to findings of hyperglycaemia, bradycardia and metabolic acidosis. A DDI was suspected between nirmatrelvir/ritonavir and verapamil. After discontinuation of nirmatrelvir/ritonavir the patient’s symptoms subsided within 4 days.48 Amiodarone has been cited among drugs that are contraindicated with nirmatrelvir/ritonavir. Sluijters et al. reported on the case of a 72-year-old patient with follicular lymphoma treated with rituximab-cyclophosphamide-doxorubicin-vincristine-prednisone, and persistent AF treated with amiodarone and receiving apixaban who had remained positive for SARS-CoV-2 for 3 months.49 Amiodarone was discontinued for 48 hours before initiating nirmatrelvir/ritonavir and restarted 96 hours after completion of the 5-day course of antiviral treatment. Nirmatrelvir/ritonavir was considered to be well tolerated and the patient was negative for SARS-CoV-2 after testing at day 7. Prospective simulations have hinted that any DDI between amiodarone and nirmatrelvir/ritonavir is possibly inflated and warrants more comprehensive clinical evaluation.50 However, at present, it is emphasised that combining nirmatrelvir/ritonavir with antiarrhythmics such as amiodarone, dronedarone, flecainide, propafenone and quinidine may have severe and potentially fatal consequences.25
A Focus on Statins
Management of risk factors for CVD is crucial and hypercholesterolaemia – one of the most important modifiable cardiovascular risk factors – is still highly prevalent in the general population.51 Considering this, statins are one of most widely prescribed drug classes.52 While most authors recommend discontinuing statins, there is some controversy. Marzolini et al. recommend discontinuing statins for at least 3–5 days following treatment with nirmatrelvir/ritonavir.30 These authors consider that temporarily discontinuing statins during nirmatrelvir/ritonavir therapy will not have any relevant therapeutic effects, but could potentially lower the risk of adverse DDIs.30 However, Abraham et al. consider that simvastatin and lovastatin have an absolute contraindication, while dose adjustments or temporary discontinuation are needed for atorvastatin and rosuvastatin.53 In contrast, pravastatin, fluvastatin and pitavastatin can be considered safer to co-administer with nirmatrelvir/ritonavir. Vuorio et al. are more cautious and consider that there may be a risk associated with temporarily withholding a statin in patients ≥65 years.54 These authors recommend substituting simvastatin or lovastatin with either pravastatin or fluvastatin.
Guidance for Avoiding Drug–drug Interactions with Nirmatrelvir/Ritonavir
The University of Liverpool has provided an updated online tool and mobile app to search for DDIs with drugs used to treat COVID-19, including nirmatrelvir/ritonavir, and a large number of co-medications.36 This easy-to-use tool provides an extremely useful resource for clinicians to check for potential DDIs with nirmatrelvir/ritonavir.36
Several societies have issued specific recommendations to guide clinicians in avoiding DDIs with nirmatrelvir/ritonavir. The American College of Cardiology published a list of DDIs with nirmatrelvir/ritonavir and commonly used cardiovascular medications.55 The guidance notes that shared decision-making should be used to consider the risks/benefits of nirmatrelvir/ritonavir therapy, while considering factors such as the risk of severe COVID-19, age, comorbidities and prior infection, as well as the possible risks of changing the cardiovascular therapy. If risk is likely to result, then it would be worthwhile to consider alternative treatment options for COVID-19.
The French Society of Pharmacology and Therapeutics has also provided recommendations for administration of nirmatrelvir/ritonavir with commonly used drugs.56 While the guidance notes that nirmatrelvir/ritonavir is a first-line option for oral treatment of patients developing COVID-19 and at risk of severe disease, it is further acknowledged that the drug has some limitations due to safety. When a DDI is expected, possible alternatives to nirmatrelvir/ritonavir include sotrovimab and remdesivir.
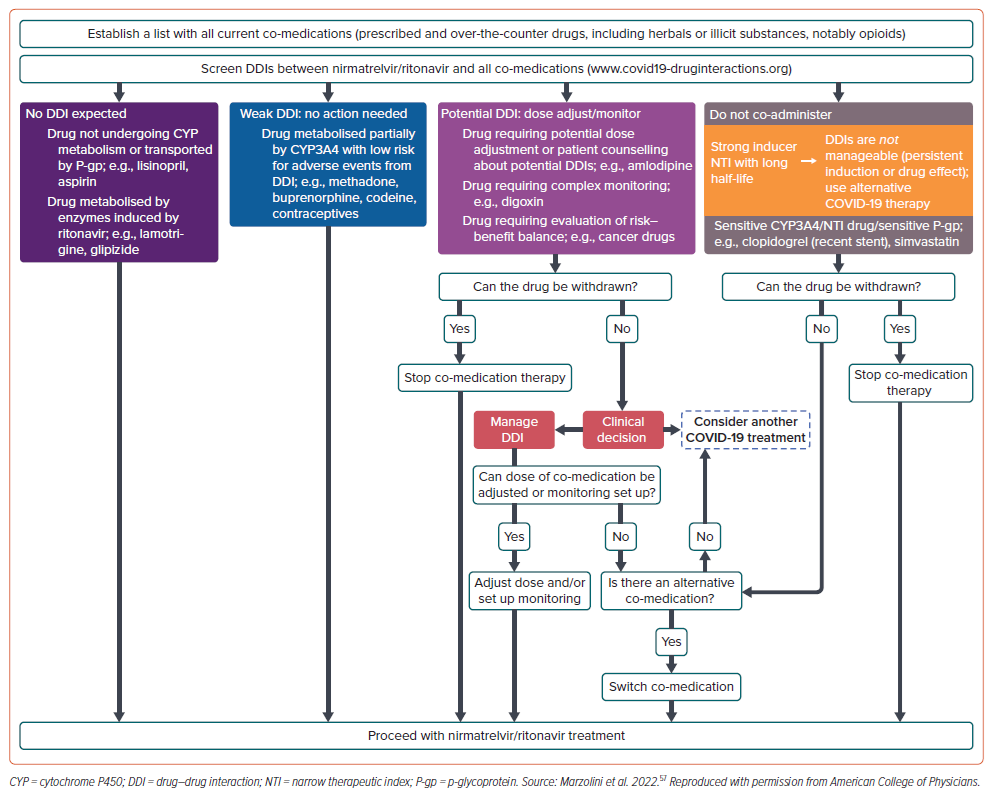
Marzolini et al. have provided specific recommendations for DDIs between nirmatrelvir/ritonavir and many drugs and drug classes.30 In line with other guidance, antiarrhythmics are not recommended with nirmatrelvir/ritonavir. It is also mentioned that potential DDIs can be expected with oxycodone requiring dose reduction. The same authors have also proposed an algorithm to assess management of nirmatrelvir/ritonavir DDIs (Figure 1).57 The algorithm takes into consideration that the inhibitory effect of ritonavir requires several days to resolve. Accordingly, if a co-medication is paused, therapy should be restarted 3 days after the last dose of nirmatrelvir/ritonavir. The algorithm is based on the premise that clinically relevant DDIs with nirmatrelvir/ritonavir can be managed in four ways: pre-emptive discontinuation of the co-medication; monitoring/dose adjustment of co-medications, even if this is usually not practicable; counselling of patients on possible symptoms and self-withdrawal of medications; or choosing an alternative treatment to nirmatrelvir/ritonavir.
Abraham et al. provide extensive guidance on cardiovascular DDIs with nirmatrelvir/ritonavir.53 The guidance also includes an algorithm to aid in decision-making when nirmatrelvir/ritonavir is needed in patients with COVID-19. In drugs with a potential interaction or contraindication with nirmatrelvir/ritonavir, temporary discontinuation should be considered. If this is not possible, alternatives to nirmatrelvir/ritonavir should be considered. If temporary discontinuation is possible and safe, then the cardiovascular drug should be stopped, or the dose reduced, upon initiation of nirmatrelvir/ritonavir and restarted 3 days after stopping nirmatrelvir/ritonavir.
Rizk et al. have proposed an algorithm to manage DDIs with nirmatrelvir/ritonavir specifically for anticoagulants.58 Briefly, if the patient is on an oral anticoagulant, it must first be decided if the patients can be off the anticoagulant for 7 days. If yes, the anticoagulant should be stopped when initiating nirmatrelvir/ritonavir and reinitiated 2 days after the combination is discontinued. If the anticoagulant cannot be reasonably stopped, if on warfarin the dose should be decreased with frequent INR monitoring or an alternative therapy to nirmatrelvir/ritonavir used such as monoclonal antibodies, remdesivir and molnupiravir. If the patient is on a DOAC, the patient can be bridged with low molecular weight heparin or an alternative antiviral can be used.
Finally, Rubina, et al. have summarised the risk of DDIs of nirmatrelvir/ritonavir with cardiovascular drugs by compiling data from six databases.59 Information on DDIs was collected on all drugs used to treat COVID-19 and antihyperglycemic agents, cardiovascular drugs and antihypertensives.
Drug–drug Interactions with Nirmatrelvir/Ritonavir in Daily Practice
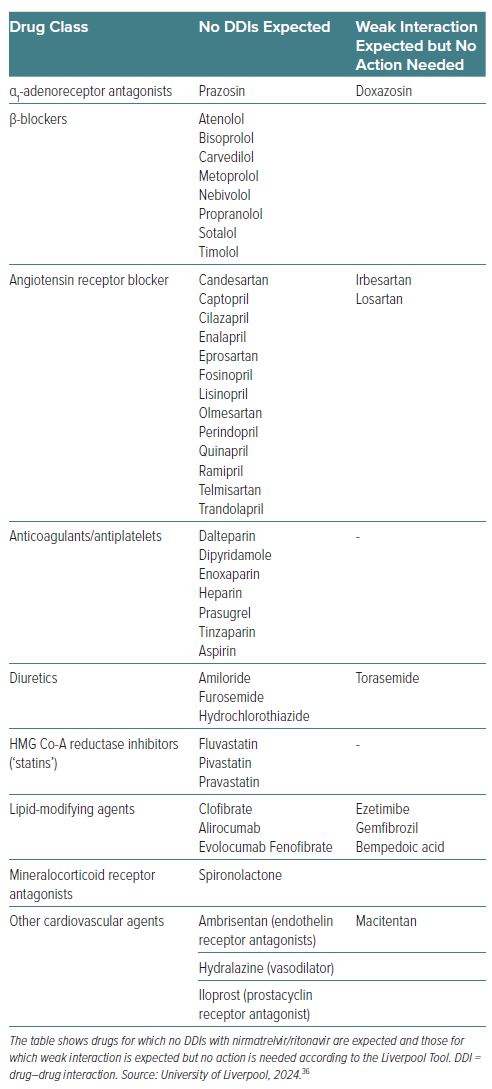
DDIs remain one of the most significant factors in achieving therapeutic efficacy with nirmatrelvir/ritonavir.59 Table 3 summarises the DDIs with several relevant cardiovascular drug classes. Avoiding and managing potential DDIs with nirmatrelvir/ritonavir requires thorough assessment and knowledge, which is particularly relevant in patients with CVD who are likely to be older and on polypharmacy. In addition, cardiovascular drugs for which no DDIs or weak DDIs are expected with nirmatrelvir/ritonavir according to the Liverpool Tool are listed in Table 4. There are many excellent resources upon which clinicians can rely to guide therapy with nirmatrelvir/ritonavir or, when needed, an alternative antiviral. Any doubts or concerns should be resolved by consulting the Summary of Product Characteristics.25 The decisional algorithm proposed by Marzolini et al. is also an excellent tool for clinicians to assist decision-making (Figure 1).57
Conclusion
DDIs are a clinically relevant issue for the treatment of COVID-19 with nirmatrelvir/ritonavir. Particular attention is needed for drugs that are predominantly metabolised by CYP3A4, are substrates of P-gp and have a narrow therapeutic index. The management of nirmatrelvir/ritonavir therapy is simplified by the short treatment course, a factor that may justify pausing the co-medication therapy. However, only a few therapies can be paused, so decisional algorithms have been proposed to manage treatment. Nevertheless, such guidance is mainly based on predicted DDIs that take into consideration in vitro preclinical data of drug metabolism and substrate recognition to drug transporters. Relevant help comes from the two decades of experience of HIV specialists with ritonavir boosting. Thus, knowledge of the pharmacokinetic characteristics of co-administered drugs represents the starting point for proper management of potential DDIs that must balance the benefit of nirmatrelvir/ritonavir to prevent severe disease against the potential risk of serious adverse events.