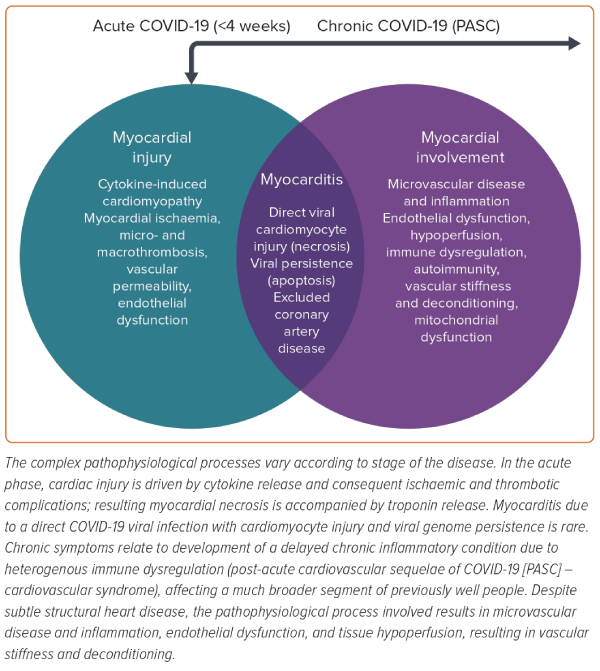
COVID-19 (due to infection with severe acute respiratory syndrome coronavirus 2) continues to exert a profound and ongoing effect worldwide. The burden of severe acute illness on healthcare systems has increased due to the growing population of patients with lingering complications of COVID-19.1 Persisting cardiac symptoms are increasingly recognised complications, now referred to as post-acute cardiovascular sequelae of COVID-19 infection (PASC).2,3 In the acute phase, cardiac injury is driven by cytokine release and the overall burden of febrile illness.4 Myocardial damage stems from ischaemic and thrombotic complications resulting in myocardial necrosis, thus often accompanied by troponin release. Patients with pre-existing cardiac conditions are particularly vulnerable. Myocarditis due to a direct viral infection is rare. Chronic symptoms relate to either worsening of pre-existing heart disease (PASC – cardiovascular disease, PASC-CVD) or delayed chronic inflammatory condition due to heterogenous immune dysregulation (PASC – cardiovascular syndrome, PACS-CVS).3 The latter affects a much broader segment of previously well people and is characterised by non-ischaemic myopericardial inflammation and only subtle structural heart disease, often undetectable by routine diagnostic tests.5 Both PASC presentations are associated with increased cardiovascular risk, reduced quality of life and disability.2
The recognition and management of cardiac involvement in the clinical setting remains a considerable challenge. Management of PASC relies on the multidisciplinary teamwork of healthcare care providers with experience in the complex nature and course of COVID-related disease. We advocate for a greater role for versatile imaging methods, such as cardiac MRI (CMR), which can inform the complex pathophysiological mechanisms underlying COVID-19-related injury.
Recognition of pre-existing cardiac conditions and early detection of post-acute complications support early implementation of guideline-directed interventions. Detection of subtle changes in previously healthy people provides reassurance as to the source of the persistent symptoms; the underlying processes may include low-grade myocardial inflammation, microvascular disease, increased aortic and ventricular stiffness, reduced stroke volume, but rarely overt global systolic dysfunction. Sensitive imaging methods are needed to detect the subtle changes in PASC-CVS, alongside considerable clinical experience in inflammatory cardiac conditions. Whereas validated treatments specific to PASC are yet to be established, early signs of cardiac impairment or ongoing chronic inflammation can guide cardioprotective intervention in those with persistent symptoms.
Pathophysiology of Cardiac Involvement Due to COVID-19
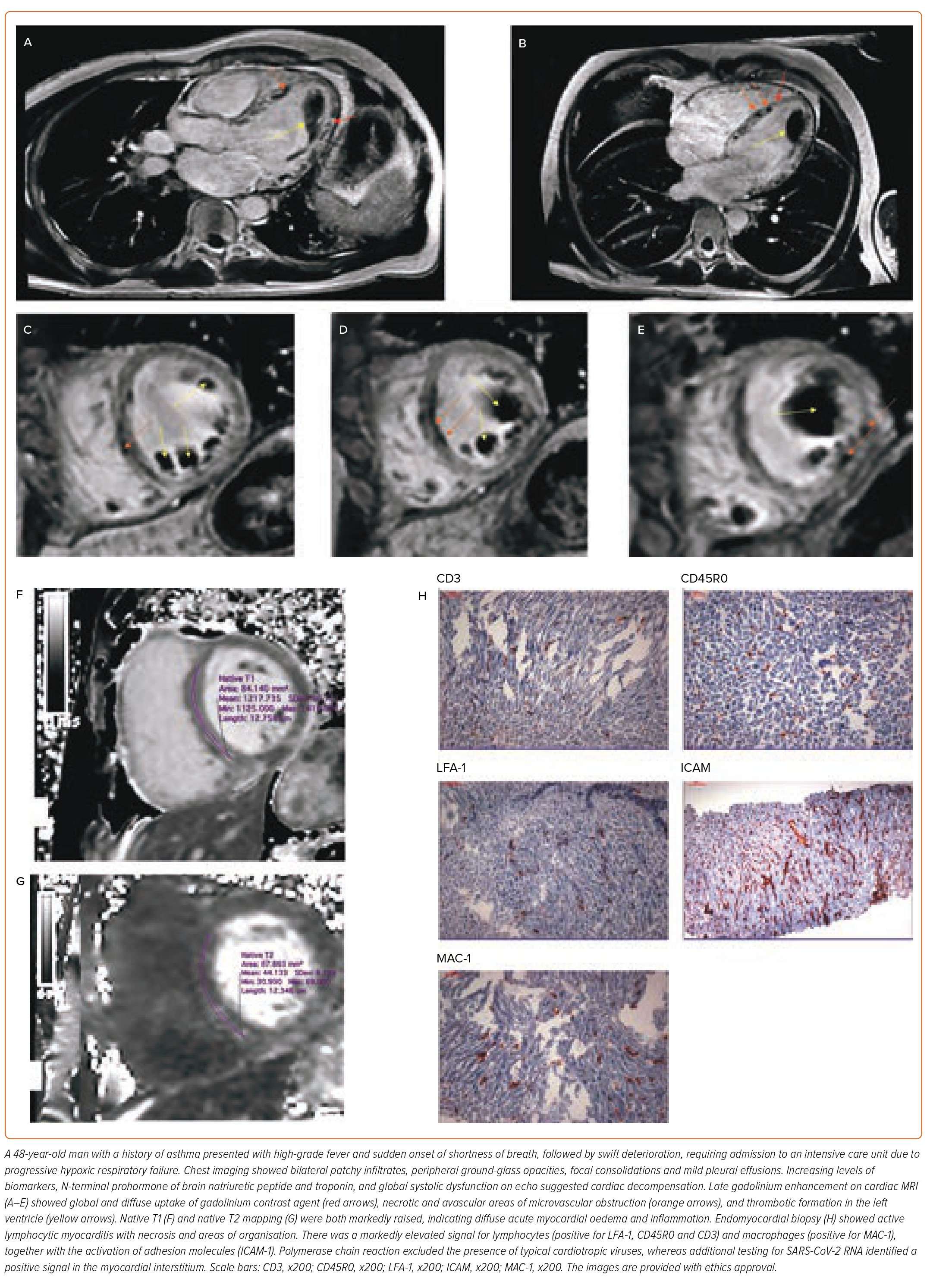
Cardiac involvement due to COVID-19 can be broadly differentiated into acute and chronic manifestations (Figure 1). The acute myocardial injury results from the systemic inflammatory response to viral illness. Driven by cytokine release (cytokine-induced cardiomyopathy), a combination of increased vascular permeability and hypercoagulation leads to impaired microcirculation, resulting in tissue hypoxaemia, micro- and macrothrombosis, and myocardial necrosis.5–7 Direct viral cardiomyocyte injury due to myocarditis is rare; endomyocardial biopsy findings include active lymphocytic myocarditis and perivascular inflammation; myocardial necrosis or viral genome is rarely found, and active replication is uncommon.8–10 The pathophysiology of myocardial injury acute COVID-19 illness has been well documented in autopsy reports and imaging case studies, including biventricular failure, and intracardiac and intracoronary thrombus formation leading to infarctions and thromboembolic showers with distal micronecrosis and cardiac decompensation (Figure 2).11,12 Non-specific lymphocyte and macrophage tissue infiltration, microvascular damage and profoundly oedematous and necrotic myocardial tissue are the hallmark of histopathological findings. In the cardiac specimens, viral RNA often co-localises with cellular infiltrate in the interstitial myocardial tissue. The clinical manifestations may range from mild symptoms with shortness of breath and chest tightness, to severe presentation with acute heart failure and cardiogenic shock. The pre-existing cardiovascular disease and risk factors, as well as a sustained cardiac injury, predispose to an increased rate of cardiac complications in the early post-discharge period.2,13
Chronic inflammatory cardiac involvement affects a much broader segment of previously well people who may have had only a mild acute illness.1,5,14 The symptoms commonly develop after a delay of weeks or months after a period of relative recovery from the acute illness; they are rarely continuous with the acute illness. Symptoms related to the cardiovascular system include exertional dyspnoea, exercise intolerance, chest tightness, pulling or burning chest pain and palpitations.1,5,15,16 Patients may note increased resting heart rate or excessive tachycardia on minimal exertion, such as standing up or walking round the house, mimicking the syndrome of postural orthostatic tachycardia (POTS). Tachycardia is often associated with shortness of breath, sensation of general weakness, dizziness, or even blackouts. Another common symptom is dyspnoea on exertion while climbing stairs or attempting slopes, despite prior high fitness levels. Low blood pressure often accompanies those with tachycardia, whereas in others elevated blood pressure is a common new finding. Sharp, pulling or burning chest discomfort, radiating across the whole chest or into the back, exacerbated by positional changes, is common in the early stages and mostly related to pericarditis, whereas deep and dull chest tightness in the aftermath of exercise or exacerbated by stress, accompanied by shortness of breath on minimal exertion, relates to microvascular disease.17–19 Although dyspnoea is often related to pulmonary involvement, lung assessments in these patients are mostly unremarkable. The overall clinical presentations may range from mild to severe cases with substantial reduction in quality of life and disability. An important systemic feature in PASC is postexercise malaise, the exacerbation or worsening of symptoms by activity, often presenting as prolonged periods of generalised weakness, fever, muscle pains, chest discomfort and headaches, often described as ‘crashes’.20,21 Crashes follow the overactivity with a time delay of hours or even days, creating an unhelpfully long feedback loop to gauge the safe level of exercise. Persistent tachycardia after exertion is an important early warning sign of clinical worsening. Crashes beget more crashes, resulting in cardiopulmonary deconditioning and worsening of exercise tolerance over time. The chronic and difficult course of recovery makes the return to professional life often unpredictable.1,22,23
The current knowledge of the underlying pathophysiology of poor health in previously well patients has been comprehensively summarised by Davis et al.1 It involves a complex form of immune dysregulation in response to viral infection, including abnormal humoral and cellular responses, with autoimmunity and primed immune mimicry, essentially a chronic inflammatory condition. The symptoms stem from tissue-level hypoperfusion due to dysfunctional small vessel capillary networks, by a combination of endothelial dysfunction, permeability and procoagulability (microclots), with impaired tissue oxygen delivery and extraction. The ensuing cellular-level changes, such as mitochondrial dysfunction, are likely to represent a cellular-level equivalent of deconditioning. The vascular denominator may explain the systemic multiorgan form of disability, from cognitive and memory issues, headaches, eye pain and vision disturbances, kidney and adrenal vascular disease, peripheral neuropathy and paraesthesia, myalgia, swelling of small joints, sleep disorders, gastrointestinal disturbances, fatigue, and so on.
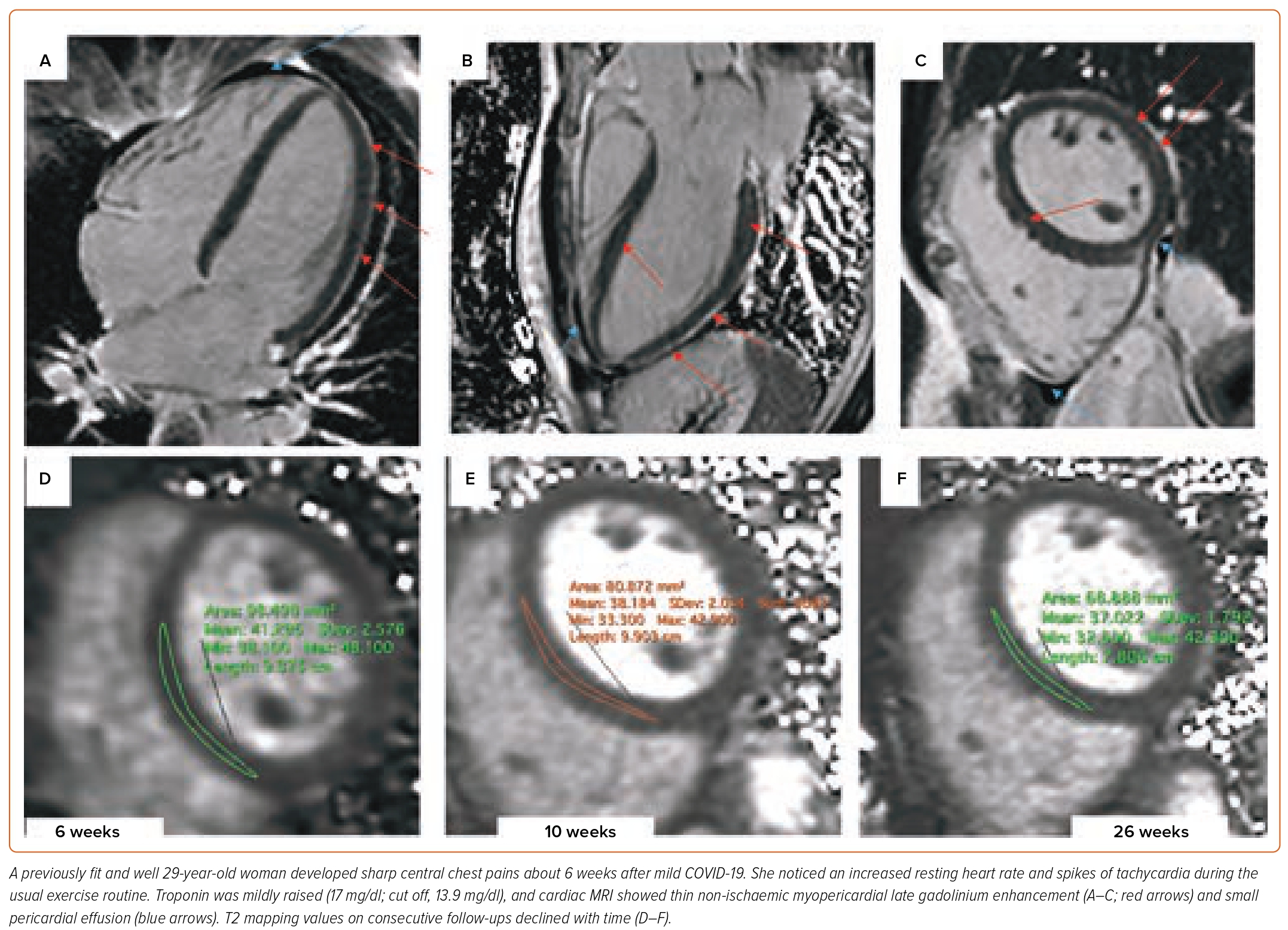
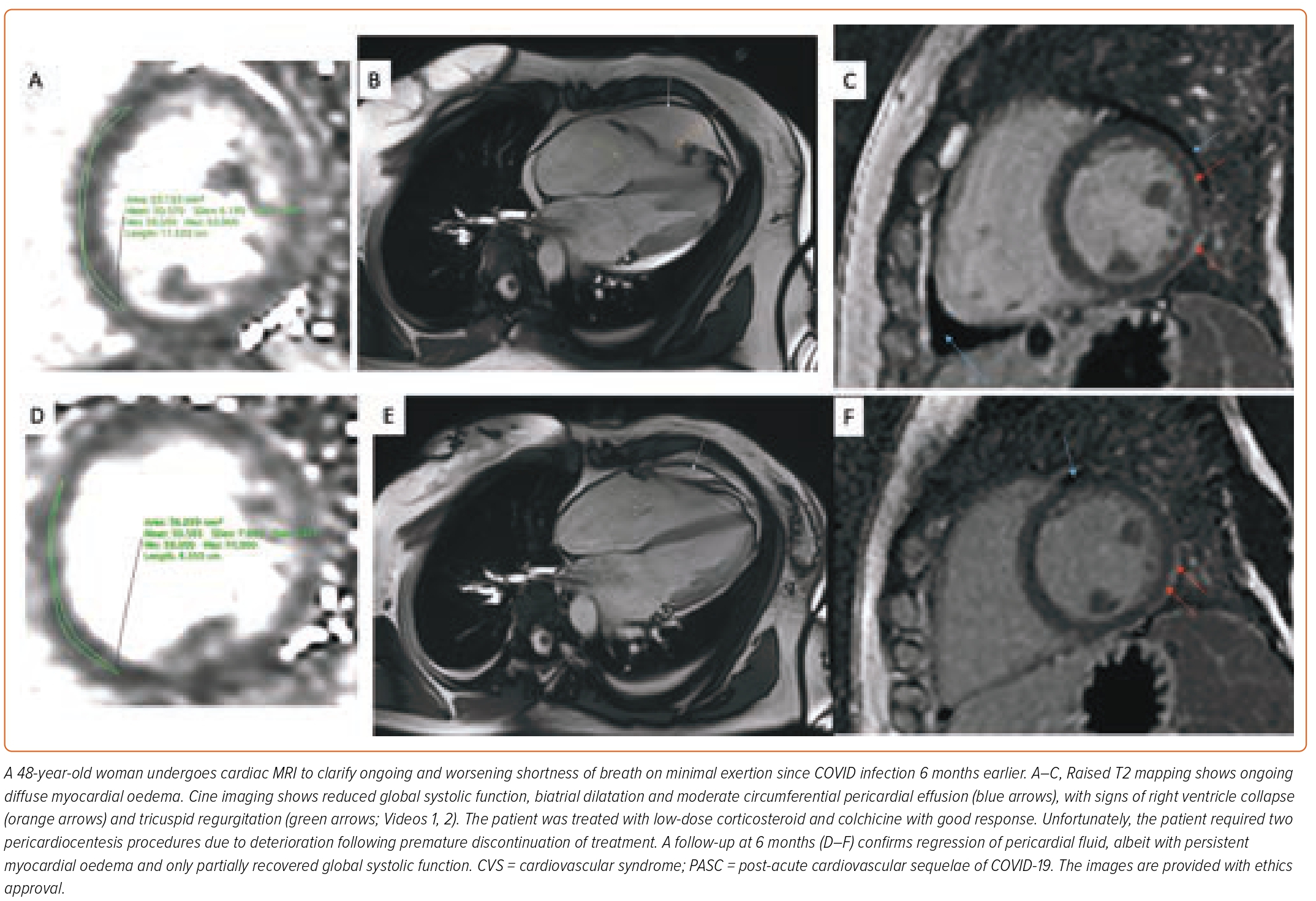
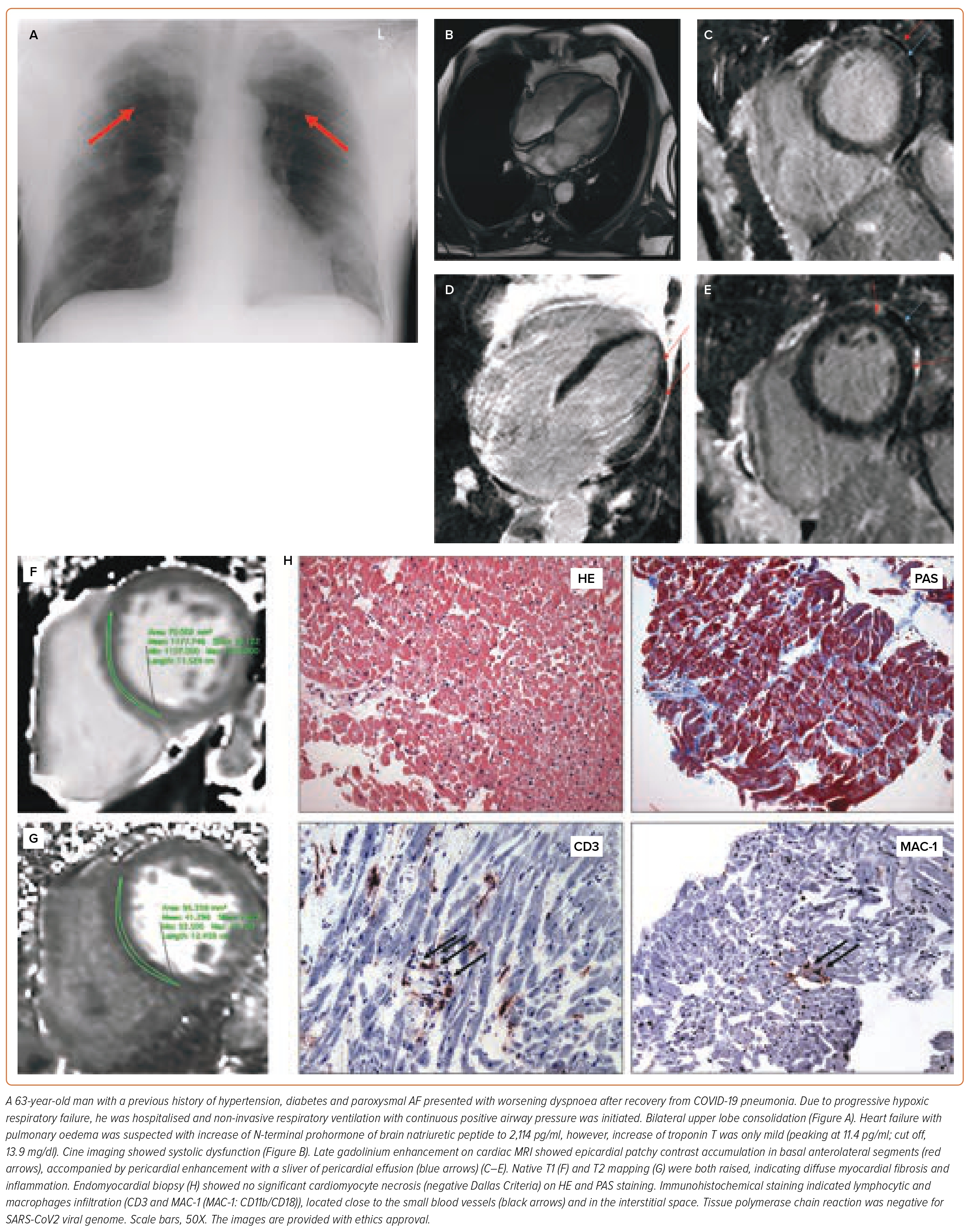
In PASC, the state of hyperdynamic circulation by tachycardia and deconditioning often masks the extent of the cardiac involvement. Cardiac biomarkers are unrevealing; normal troponin is concordant with the absence of direct cardiomyocyte damage. Low intravascular volume and lack of exertion prevents increased wall stress, necessary for NT-proBNP (N-terminal prohormone of brain natriuretic peptide) release (Figures 3 and 4). Imaging may help to uncover small pericardial effusion or even considerable pericardial effusion with tamponade (Figure 5, Supplementary Material Videos 1 and 2). Structural measurements indicate normal dimensions and often supernormal systolic function, especially in women with low flow velocities. Sensitive serial volumetric measurements of stroke volume may help to detect gradual reduction, often first in the low normal range. Tissue characterisation is crucial to uncovering the underlying inflammatory phenotype, including the low-grade diffuse myocardial tissue oedema, thickened pericardial layers, and epicardial and intramyocardial late gadolinium enhancement.5 These abnormalities can be detected as early as 2 weeks after the infection and may persist for several months.
Necrotic, infarct-type myocardium due to direct cardiotropic viral damage is rare in PASC patients.24 Several large studies in athletes returned negative results based on the Lake Louise criteria, which can detect infarct-like myocarditis but not diffuse forms of myocardial inflammation.18,25,26 Furthermore, these studies evaluated competitive athletes for the presence of myocarditis (clinical or subclinical) based on local protocols and clinical interpretation with no standardisation of scanning methodology or core-lab reads. These studies nonetheless highlighted that the diagnostic algorithms that preselect patients for CMR using the findings of less sensitive methods, such as ECG or echocardiography, return a very low yield of overall confirmation (3%).18 This increased fourfold when CMR was used as the first-line diagnostic tool.26 The use of sensitive methods early in the diagnostic process is thus crucial. Understanding the limitations of the Lake Louise criteria in detecting diffuse disease is also important. PASC shares phenotypical commonality with cardiac manifestations in chronic systemic conditions in which diffuse myocardial involvement due to vascular inflammation is well described, including hypertension and diabetes, lupus myocarditis, systemic sclerosis, and chronic post-viral syndromes due to, for example, Epstein–Barr virus and HIV.27–36
Management Strategies in Patients with PASC
Medical management of PASC relies on expert knowledge and a deep understanding of the complex nature and unpredictable course of the disease. This insight helps to gauge the deployment of the diagnostic methods and the available treatment options. In unwell patients, the diagnostic strategy should be directed towards (but not end with) screening for overt structural heart disease, signs of cardiac decompensation, right-sided ventricular strain, tamponade or vascular emergencies.3 Serial cardiac biomarkers may raise a hint of potential deterioration, while rising brain natriuretic peptides herald the onset of heart failure, and troponin release reflects myocardial injury and is the hallmark of a high risk of complications in the short term. Thromboembolic complications, such as pulmonary embolism, must be considered early in patients with persistent tachycardia, desaturation and signs of right-sided heart dysfunction on imaging. Although fulminant myocarditis is rare, endomyocardial biopsy could be considered in cases of clinical deterioration despite intensive treatment in patients with no evidence of obstructive coronary artery disease.
In stable symptomatic PASC patients, we advocate for sensitive diagnostic methods, such as CMR, which can inform on the complex pathophysiological mechanisms underlying COVID-19-related disease. The presence of pre-existing ischaemic heart disease or worsening myocardial ischaemia can be established using myocardial perfusion imaging to clarify the need for revascularisation. In patients with symptomatic heart failure, CMR provides useful information on the underlying aetiology, differentiating between an ischaemic origin or non-ischaemic cardiomyopathy by the patterns of scar on late gadolinium enhancement imaging. In patients with PASC-CVS with chronic inflammatory syndrome but no structural heart disease, the parallels to known chronic vascular syndromes include mechanistic and phenotypical insights, as well as an overall lack of targeted cardiovascular diagnostic and treatment strategies.22,36–38
In PASC-CVS the interpretation of findings must reflect the sensitivity of the diagnostic method, and account for its analytical thresholds. Echocardiography and CMR can efficiently exclude structural heart disease in most hands. However, to detect more subtle changes that underlie the persistent symptoms in PASC-CVS, the use of advanced sensitive imaging methods is necessary. The underlying processes include low-grade myocardial inflammation, microvascular disease, increased aortic and ventricular stiffness and reduced stroke volume, but rarely overt global systolic dysfunction. Realistically, the methods to detect these findings are neither robust nor a standard part of cardiology clinical practice; therefore, considerable clinical experience, as well as imaging expertise in inflammatory cardiac conditions, is required. Clinical experience in inflammatory cardiac conditions is crucial; most doctors train their diagnostic expectation on the model of ischaemic heart disease with an overt and well-defined problem at the point of the maximal symptoms.39 As in diabetes or hypertension, structural heart disease in the context of inflammatory disease develops only after years or decades, with much subtler findings and changes in-between. Particular care must be taken in the evaluation of athletes to avoid misinterpretation of physiological structural changes frequently observed in this group of patients as pathological findings.3,40 Standard cardiac tests may be complemented by cardiopulmonary exercise testing and 6-minute walk test to help objectivise the overall functional disability and monitor improvement. Respiratory assessments and screening for classical rheumatological and neurological autoimmune conditions to clarify the overlapping symptoms are recommended.
Patients with PASC-CVD require proactive identification of cardiac complications and intensified guideline-directed interventions. Ongoing poor health and persistent symptoms, in part due to newly accrued cardiac injury during the acute illness, necessitate regular assessments and intensified guideline-directed cardiac care. The onset of new symptoms after recovery from the acute illness may also signal latent pre-existing cardiac conditions. Patients with hypertension, diabetes and metabolic syndrome are particularly at risk of cardiac complications, including supraventricular tachycardias or AF or development of heart failure.41 Early concerns regarding the possible negative synergies between COVID-19 and renin–angiotensin–aldosterone system blockers have been replaced by the evidence that withdrawing these essential medications can, in fact, be harmful.42–44
Currently, there is no validated targeted treatment for PASC-CVS. Most patients will benefit from better understanding of the underlying disease, lifestyle adjustment and activity management. Subclassification of the activity into physical, mental or social/emotional can be helpful to avoid the pitfalls of underestimation.21 Simple records of daily life are increasingly supplemented with digital apps that include heart rate tracking and similar measurements. These are helpful tools of self-observation, building awareness of the current limitations, tracking progression towards resilient stages, as well as the opposite, detecting the warning signals of imminent deterioration. Wearable devices are particularly helpful to gauge the activity through heart rate, which can be used to guide its intensity (the concept often referred to as ‘pacing’) by keeping the heart rate below 110–120 BPM. Home-based self-directed or guided breathing and floor-based stretching exercises, adjusted to remain below the crash threshold, are a good start to preserve strength and provide a daily self-assessment. Stepwise prolongation of the low-intensity exercise enables a slow but sustained, quantifiable improvement. High fluid and salt intake are an important measure to replenish intravascular volume and improve peripheral tissue perfusion; while compression stockings improve the venous return. A healthy diet, low in animal fats, with broad vitamin supplementation within the recommended daily doses, strict avoidance of recognised myocardial toxins, such as alcohol or energy drinks, as well as too much coffee due to its dehydrating effects, is also helpful. Expectation management of oneself and others is crucial, given the total absence of control over the speed of recovery.
Management of cardiovascular risk factors remains an important priority in these patients due to possible additive vascular effects. Targeted vasculoprotective treatments have an established role in chronic inflammatory conditions, however, they are yet to be established in the case of chronic COVID-19 (MYOFLAME-19, NCT05619653).27,28,45 Owing to the relative hypovolaemia in most patients, the hypertension treatment threshold is likely to be lower. The evidence for routine antithrombotic treatment in PASC patients is also missing. Tachycardic patients may benefit from symptomatic heart rate control: selective β-blockers, such as nebivolol and bisoprolol, are usually effective in reducing symptoms due to tachycardia. However, ivabradine is more suitable for often hypotensive patients. In patients with profound POTS-like symptoms, a trial of low-dose fludrocortisone may also be considered. Patients with chest pains due to pericarditis and pleuritis will benefit from early initiation of low-dose colchicine or corticosteroid treatment. High doses of these two medications, as well as ibuprofen, are usually poorly tolerated owing to many side effects, including excessive tachycardia (steroids), gastrointestinal symptoms (steroids, colchicine, ibuprofen), or kidney injury (ibuprofen). Pericarditis may be persistent, and treatment is often needed for more than 6 months; it is prone to reoccur with premature discontinuation or reinfection. The benefit of specific immunosuppressive treatments and procedures has not been formally established.
Conclusion
Cardiac complications are an increasingly recognised complication of COVID-19 and convey a high burden of morbidity and mortality. Cardiac involvement can be broadly differentiated into acute and chronic manifestations. The acute cardiac involvement is cytokine-driven and relates to ischaemic and thrombotic complications resulting in a wide range of myocardial injuries and/or necrosis. Chronic symptoms relate to either worsening pre-existing heart disease (PASC – CVD) or delayed chronic inflammatory condition due to heterogenous immune dysregulation (PASC – CVS). The latter affects a much broader segment of previously well people and is characterised by non-ischaemic myopericardial inflammation and subtle structural heart disease, often undetectable on routine diagnostic tests. Both PASC presentations are associated with increased cardiovascular risk, disability, and reduced quality of life. The recognition and management of cardiac involvement in this clinical setting remains a considerable challenge.