Epidemiology
The survival of patients with congenital heart disease (CHD) has improved dramatically over the past decades, due to continuous advances in medical care and surgical techniques. More than 85% of these individuals reach adulthood and there has been an estimated 63% rise in this population since 2000.1–3 Therefore, physicians must now deal with potential consequences derived from the natural progression of the disease, late sequelae from previous interventions or acquired conditions.4
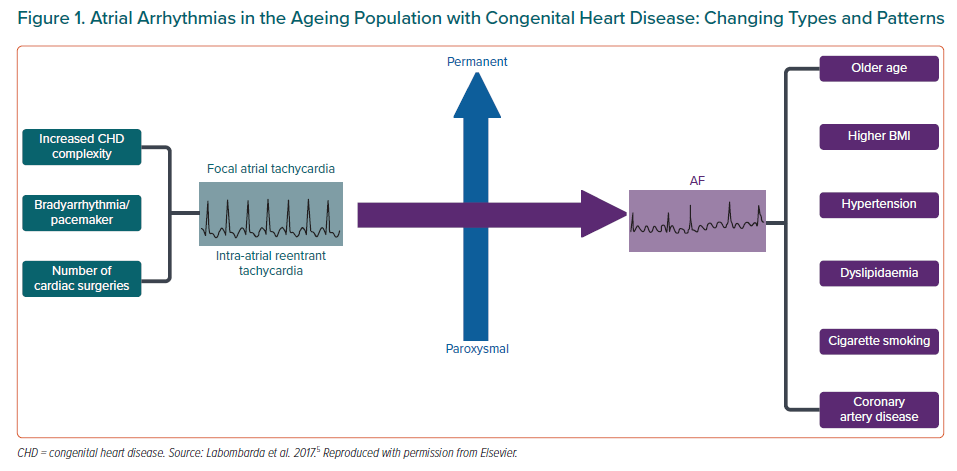
Atrial arrhythmias are the most common complication in adults with CHD and they are the leading cause of morbidity and hospital admissions. Although intra-atrial reentrant tachycardias (IART) are the most frequent presenting type of arrhythmia, the incidence of AF is increasing as patients age, surpassing IART in people over the age of 50 years.5 Furthermore, AF in CHD patients tends to develop at a younger age, with a markedly increased risk when compared to their non-CHD peers.6 Globally, IART accounts for the majority of presenting arrhythmias in patients with complex cases of CHD. In simpler lesions, both IART and AF have a similar prevalence.5
Patients with certain conditions, such as atrial or atrioventricular septal defects, Ebstein anomaly, tetralogy of Fallot, univentricular hearts, left-sided obstructive lesions and pre-existing pulmonary hypertension, are particularly prone to developing AF.7–9
As the CHD population continues to age, it is likely that AF prevalence and other arrhythmic issues will continue to increase in the next decades. It is of utmost importance that all physicians involved in the care of patients with CHD, including paediatric and adult cardiologists and electrophysiologists with expertise in CHD, collaborate to ensure appropriate patient management.3,10,11
Pathophysiology
Although the specific mechanisms of AF in the non-CHD population remain to be elucidated, ectopic activity (mainly rapid firing from the pulmonary veins) and reentry play important roles in its initiation and perpetuation.12,13
In CHD patients, both conditions can be favoured by numerous reasons. First, triggers outside the pulmonary veins, including the right atrium or the superior vena cava (right or left persistent), seem to be relevant in this population.14–17 Second, structural remodelling through atrial fibrosis from previous corrective or palliative surgeries, added to atrial enlargement caused by residual septal defects, valvular disease, ventricular dysfunction or acquired conditions, promote conduction delay and alter ion channel function, facilitating reentry.13,18,19 Finally, chronic atrial pressure and/or volume overload favour triggered activity, causing premature beats or focal atrial tachycardia (AT) that, in turn, can also trigger AF.12,20
Although the risk factors related to AF in CHD patients are common to the general population, including age, hypertension and acquired cardiovascular conditions, the described predisposing factors may contribute to AF development at a younger age (Figure 1).5 Nevertheless, research into AF pathophysiology in this population is still scarce and further studies are warranted to better understand the underlying mechanisms and determinants of AF in CHD patients that could potentially lead to improved management.
Clinical Impact of AF
Three main potential consequences may result from AF development in CHD patients: heart failure (HF), stroke and mortality. HF is the most frequent complication, with an estimated prevalence of about 11% in young CHD patients; its risk sharply increases (up to 11 times) compared to patients with CHD and no AF and in turn HF increases the risk of atrial arrhythmia recurrence.6,21 HF has also been consistently the strongest independent predictor of ischaemic stroke in this population, especially in young patients and in the first 3 years after onset.22,23
Ischaemic stroke is a dramatic and disabling complication of AF, as neurological sequelae may be permanent in up to 25% of all cases.24 Previous data have shown that young patients with CHD have a 10-fold increased risk of stroke compared to the general population. AF, HF and traditional cardiovascular risk factors independently contribute to increase this risk. The highest cumulative incidence corresponds to the more complex conditions, such as Fontan circulation and cyanotic CHD, but patients with left-sided lesions and uncorrected pre-tricuspid left-to-right shunts are also particularly at risk.23,24
Mortality rates are also greater in this patient group and this derives from AF complications. Data from more than 38,000 patients with CHD from Quebec, Canada, have shown an almost 50% increase in the risk of overall mortality in patients with atrial arrhythmias.25 Some may be related to thromboembolic events, but AF, as other atrial arrhythmias, may also contribute to sudden death.26 Fast ventricular rates may provoke ischaemia because of a mismatch in oxygen demand and supply, which can induce ischaemia-related ventricular arrhythmias, mostly in patients with impaired systolic function or single or systemic right ventricle.27–29
Most patients present with paroxysmal AF, although evolution to persistent or permanent forms is common.5 Teuwen et al. observed a rapid progression in 26% of subjects after only 3 years from the first episode. These patients, usually with more complex CHD, developed AF at younger ages. Moreover, approximately one-third had coexistence of other regular atrial arrhythmias, which generally preceded AF.30 Finally, AF may appear in the long term even after successful ablation of IART. In a cohort of repaired atrial septal defect (ASD) patients, 30% developed AF during the follow-up, 25% of them requiring AF management.31
Some CHD, such as ASD, tetralogy of Fallot, Ebstein anomaly, heterotaxy syndromes and Fontan palliation, carry a higher likelihood of the patient developing AF.32 Reasons for this are speculative and are likely to be based on many factors. First, patients with simpler forms are more likely to live longer and are more commonly exposed to classical risk factors and develop acquired cardiovascular conditions. Second, it is likely that specific haemodynamics have a different effect on atrial remodelling. In ASDs, significant structural remodelling in the left atrium, such as reduced voltage, delayed and heterogenous conduction, has been described.33 It seems reasonable to believe that similar remodelling may be observed in other CHD, such as Ebstein anomaly or left-sided lesions, that may be associated with atrial enlargement. Third, other predisposing factors, such as sinus node dysfunction, are more common in some CHD, such as heterotaxy syndromes, as opposed to others.
AF risk in patients with ASD, despite defect closure, remains higher than in a non-CHD matched population. Early closure, either surgical or percutaneous, reduces this risk compared to late repair.34–37 Patients with tetralogy of Fallot are susceptible to developing atrial arrhythmias in the long term, with AF increasing in prevalence, predominantly in those with concomitant left-sided lesions.38 In subjects with Ebstein anomaly, although supraventricular tachycardias mediated by accessory pathways are the cornerstone arrhythmia, atrial tachycardias (including AF) can be encountered in 25–65% of the patients.39,40 Finally, subjects with Fontan physiology, depending on the type of surgery they have had, 10–50% may suffer atrial arrhythmias as a late complication, presenting initially with IART or focal AT that can evolve to AF. It is also a high-risk condition for thrombotic complications.41–43
Acute Management
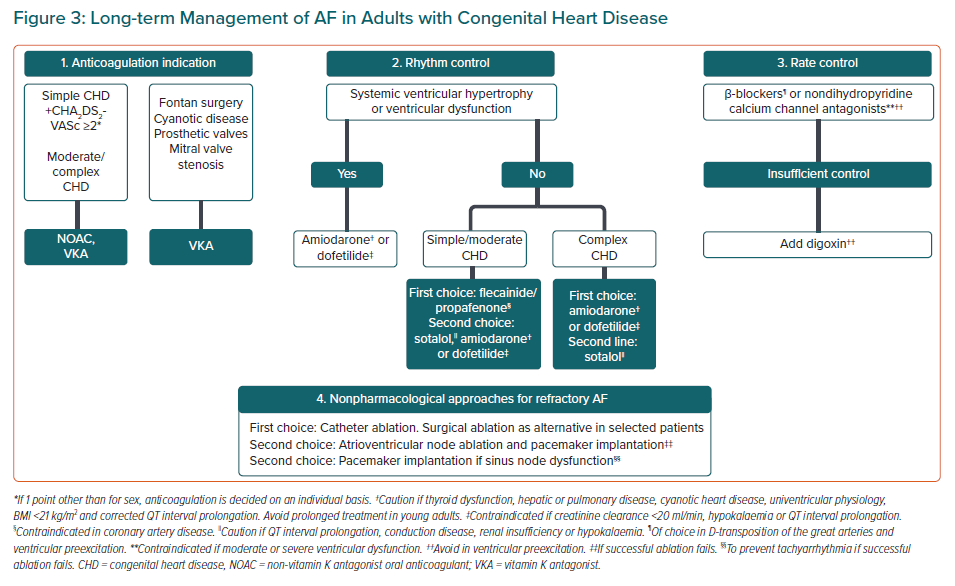
AF has a negative haemodynamic effect in CHD patients because of the loss of atrioventricular synchrony and, if present, the fast ventricular rate. The restoration of sinus rhythm should be considered the first option in the majority of cases. Haemodynamically unstable patients must undergo urgent electrical cardioversion (CV), regardless of the duration of the arrhythmia and their anticoagulation status.44 In a non-urgent setting, a strategy of 4 weeks of anticoagulation with direct CV has proven to be safe in low-risk patients with CHD.45 Although classically no anticoagulation was recommended for acute CV if AF began in the previous 48 hours, recent data suggest that patients with predisposing factors (CHA2DS2-VASc ≥2) have an increased risk of thromboembolic events, especially if delaying CV more than 12 hours after onset. Periprocedural anticoagulation in this context reduced this risk by 82%.46,47 Therefore, irrespective of AF duration, in subjects with high-risk conditions, such as patients with mechanical prosthesis, moderate to severe atrioventricular stenosis, a prior history of thromboembolism, moderate to severe CHD, or if it is more than 12 hours since AF began and CHA2DS2-VASc ≥2, transoesophageal echocardiogram (TOE) or 3 weeks of anticoagulation before CV is recommended. A combination of both may be considered in this context as a Class IIbC suggestion in the recent European AF guidelines (Figure 2).11,48,49
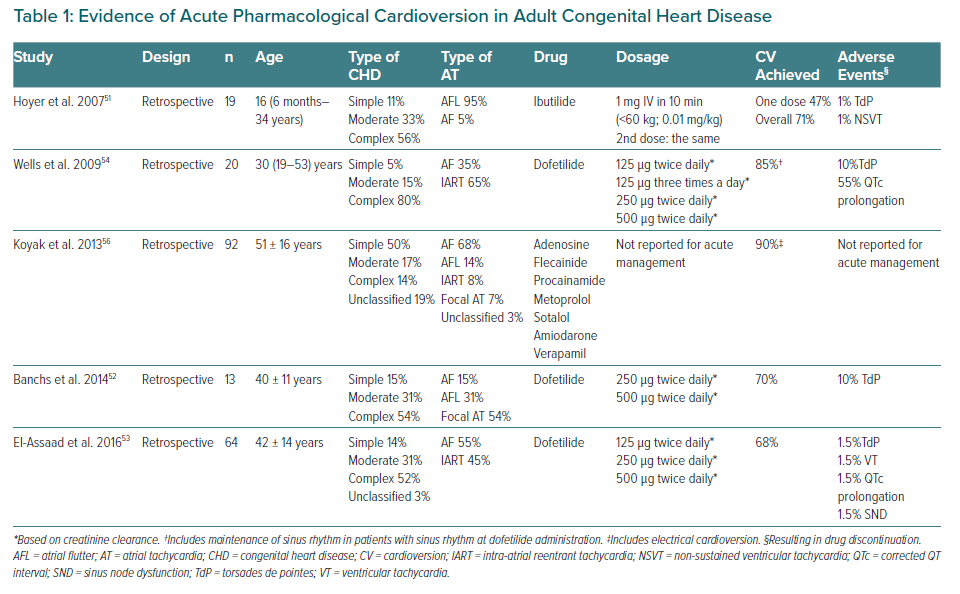
Choosing between electrical or pharmacological CV should be done on an individual basis. Although pharmacological CV avoids sedation, evidence of antiarrhythmic drugs in CHD is limited and proarrhythmic effects may be of particular concern.50 Literature about efficacy or safety of acute CV for atrial arrhythmias in this population is scarce. Most experience has been reported with ibutilide or dofetilide, which are not available in Europe.51–54 The procedure should be monitored with resuscitation equipment available, as the risk of torsades de pointes is estimated at about 4.3%.32,55 Amiodarone may also be a reasonable option.56 A summary of the available evidence is presented in Table 1.
Long-term Management
The mainstream long-term therapy for AF includes anticoagulation, if indicated, in addition to pharmacological and interventional strategies to prevent arrhythmia recurrence.
CHD patients are not represented in rate versus rhythm control randomised trials and no direct comparison between the two strategies has been performed in this population. Considering the deleterious effects of losing sinus rhythm, both the American and European consensus documents for arrhythmia management in adults with CHD advocate a rhythm control strategy as the initial approach (IIaC and IC recommendations, respectively).32,44 Maintenance of atrioventricular synchrony is especially relevant in complex conditions such as univentricular physiology or dysfunctional systemic right ventricles.14 Nevertheless, before a definitive management strategy is established, reversible causes of AF should be sought and corrected if possible – such as residual shunts, obstructive or regurgitant lesions, reduced left ventricular function and myocardial ischaemia – as part of AF management to avoid recurrences. If the patient develops bradycardia-tachycardia syndrome, pacemaker implantation may be considered to prevent tachyarrhythmia if successful ablation fails or is not feasible (Class IIaC recommendation).10
Achieving this goal poses several challenges. First, evidence about antiarrhythmic drugs in CHD is limited and comes mainly from retrospective studies. Second, despite its use, the recurrence rate is high so ablation emerges as an appealing alternative and is being increasingly performed; however, its experience in the CHD setting is still limited, and the underlying lesion or prior interventions may make it difficult to access the ablation targets. Finally, little is known regarding pre-emptive measures that could reduce the risk of AF, particularly in complex patients with cyanotic conditions, restrictive physiologies, univentricular hearts or systemic right ventricles.30,57–59
Rhythm Control
Antiarrhythmic Drugs
Several aspects must be considered when choosing an antiarrhythmic drug in adults with CHD, including ventricular function, conduction disturbances, such as sinus node dysfunction, impaired AV nodal conduction, pregnancy plans, comorbidities such as kidney or hepatic failure that may affect pharmacokinetics, and concomitant therapies with potential significant interactions.
Similar to the non-CHD population, Class I antiarrhythmic agents are not recommended in patients with coronary artery disease or moderate to severely impaired systolic function of a systemic or sub-pulmonary ventricle because of potential increased mortality, so they are reserved for simple or moderate CHD without any of these factors.11,60–63
Class III agents seem to be more effective than any other class in preventing atrial arrhythmia recurrences. Nevertheless, adverse events, mostly described with amiodarone, are common and lead to discontinuation of therapy in a high proportion of patients, especially if required in the long term.56,64 Thyroid dysfunction, mostly amiodarone-induced thyrotoxicosis, is the most frequent complication, potentially causing exacerbation of tachyarrhythmias. Patients with Fontan circulation are particularly vulnerable because of their altered hepatic metabolism. Additional high-risk subgroups are women, people with cyanotic conditions, other univentricular physiologies and a BMI <21 kg/m2.65–67 The incidence of induced bradyarrhythmia seems to be low.64
Complete or partial control of refractory tachyarrhythmias can be achieved with sotalol, especially if combined with non-pharmacological approaches.68 However, because of its proarrhythmic effects, it should not be used in patients with significant ventricular systolic dysfunction.
Extrapolating data from the general population, dronedarone is not recommended in people with moderate or complex CHD, HF or at least moderate ventricular dysfunction because of the concerns of augmented mortality, worsening of heart failure and stroke.69,70
In summary, Class I agents may be considered as the first-line therapy for the simple/moderate forms of CHD without significant ventricular hypertrophy or dysfunction or myocardial scarring. Otherwise, amiodarone with a IIaC recommendation or dofetilide with a IIaB recommendation can be used. Long-term therapy with amiodarone should be avoided in young adults because of common side-effects. Attention should be paid to dofetilide contraindications (creatinine clearance <20 ml/min, hypokalaemia, QTc >440 ms) and cardiac monitoring for 72 hours after initiation is advised (Figure 3).32,44
Catheter and Surgical Ablation
The disappointing results and the aforementioned limitations of antiarrhythmic drugs, together with the significant improvements in technical aspects of ablation procedures and the growing evidence for its use in the general population, has resulted in an expanding use in CHD patients.71,72 Thorough preprocedural planning is mandatory to minimise complications and maximise success. Documenting arrhythmia to rule out other atrial arrhythmias, review of individual anatomical features with multimodality imaging and careful evaluation of vascular accesses are of utmost importance.73–75 Transseptal puncture may pose additional challenges in CHD patients because of distorted anatomy or the presence of atrial surgical patches or closure devices.76 Puncture through surgically or percutaneously repaired ASDs can be difficult but it’s feasible in most cases. Access can be obtained through the native septum or, if not possible, through the device.77 Occasionally, residual shunts may facilitate access to the left atrium. Intracardiac echocardiography may be helpful to guide the transseptal puncture and detect residual shunts.
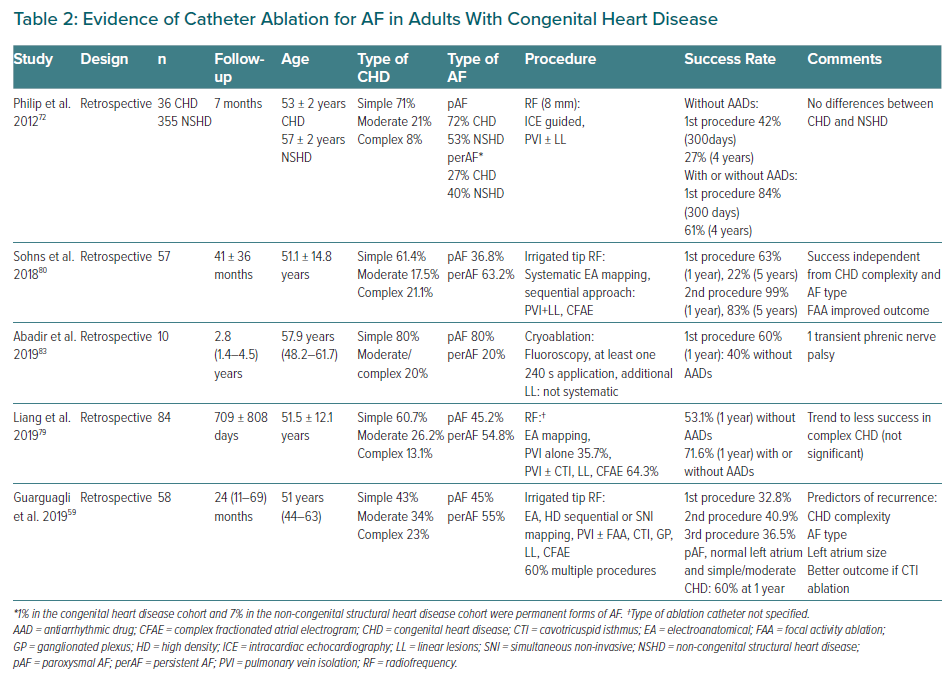
Most of the reported experience with catheter ablation is related to radiofrequency. Procedures should be performed using 3D mapping technology (Class Ib recommendation) and irrigated-tip catheters for ablation (Class IIaB recommendation).32,44,78 The role of novel mapping tools like high-density or rotor mapping remain to be elucidated. Additional ablation targets beyond the pulmonary veins may be considered, especially in redo procedures, including additional lines, such as atrial roof, mitral or cavotricuspid to pulmonary vein isolation isthmus, or non-pulmonary vein triggers.79,80 Success rates at 1 year with or without antiarrhythmic drugs range from 33–84%. Difficulties accessing the left atrium with reduced catheter contact, the inability to deliver transmural lesions because of excessive wall thickness or the less relevant role of the pulmonary veins may account for some of the reasons of high recurrence rates in this population.14,81,82
Initial experience with cryoablation has proven safe, with high acute success but also modest long-term outcomes.83 Evidence for catheter ablation in CHD is summarised in Table 2. Despite being considered a first-line treatment for other atrial arrhythmias, the increased complexity of the procedure and the modest results, the limited experience and the peculiarities of these patients have translated to a IIbC recommendation for catheter-based pulmonary vein ablation in patients with drug-refractory AF.10 It should only be performed in centres with experience in both AF ablation and CHD management.11 The role of AF ablation in this specific setting needs to be further explored. Research to shed light on predictors of success or failure in order to detect appropriate candidates for AF ablation, or targeting AF precursors to prevent AF, such as focal atrial tachycardias or IART is warranted.
Finally, surgical ablation (the Cox-Maze procedure) at the time of congenital heart defects repair – surgical ASD closure, Ebstein anomaly and Fontan conversion – in presence of symptomatic AF is recommended (Class IIaC), and even prophylactic surgical ablation in patients at high risk of AF, can be considered (Class IIbC).11,32 Extensive Maze procedures are recommended to avoid the potential proarrhythmic effects of limited atrial Maze techniques.84 Surgical management of atrial arrhythmias at the time of CHD surgery does not seem to increase complications and has reasonable outcomes, with arrhythmia-free survival of 75% at 6 year follow-up.85,86 This has made some groups advocate for systematic surgical management of preoperatively diagnosed atrial arrhythmias at time of surgery, because defect correction alone will not significantly diminish arrhythmic risk. Nevertheless, it is important to note that surgical access in patients with prior sternotomies may be difficult and limited.
AF surgical ablation without other concomitant surgery can be considered on an individual basis after repeated catheter ablation failures, absence of percutaneous access to ablation targets or if alternative approaches such as rate control or atrioventricular node ablation and pacemaker stimulation are not desirable.
Rate Control
Whenever rhythm control strategy is abandoned, rate control is essential to avoid secondary development of tachycardiomyopathy or worsening heart failure.26 For this purpose, both β-blockers and nondihydropyridine calcium channel blockers can be used. Some data support the preference of β-blockers due to a protective effect against ventricular tachycardia in specific situations such as transposition of the great arteries corrected with atrial switch.27,29 Digoxin is usually left as a second choice, mostly in combination with one of the other drugs (Figure 3).87 There is no specific data in CHD patients regarding the optimal target heart rate, so following a similar strategy to the general population seems reasonable.88,89
Atrioventricular node ablation and pacemaker implantation might be considered in case of uncontrolled ventricular rates despite the association of multiple rate control drugs and should be reserved as the last resort.11,90 Adequate transvenous access for pacing and ablation should be confirmed. The atrioventricular node may be difficult to locate in CHD related to anatomical variations or after reparative surgeries.
Anticoagulation
Standard scores used to guide oral anticoagulation do not consider CHD and have not been validated in this specific population.91 Besides, stroke risk is much higher in CHD patients. A recent multicentre retrospective study found that CHD complexity was an independent risk factor for thromboembolic events. In this cohort, the CHADS2 and CHA2DS2-VASc score did not correctly identify patients at risk as they were young and did not have other conventional risk factors.58 Current recommendations advocate taking into account disease complexity in addition to traditional scores. Thus, patients with moderate (Class IIaC recommendation) or complex (Class IC) CHD and sustained or recurrent AF should be on long-term oral anticoagulation. For patients with simple forms of CHD and without valvular prosthesis the decision on anticoagulation should be based on the CHA2DS2-VASc and HAS-BLED scores (Class IIbB).92,93
Since their appearance, non-vitamin K antagonist oral anticoagulants (NOACs) seem an appealing option for CHD patients. Recent evidence suggests that they are safe even in moderate and complex forms of CHD, including those with significant valvular lesions and bioprosthetic valves.94,95 Subjects with Fontan palliation are still a challenge, as thromboembolic episodes and significant bleeding have been described in this group of patients despite NOAC therapy in the multicentre prospective NOTE registry but further research is warranted to expand their use in this subgroup of patients.96 The European guidelines on the management of adult CHD recommend NOACs as the first treatment option for CHD patients, excluding those with Fontan physiology, chronic cyanosis, mechanical prosthetic valves or significant mitral stenosis.10
Left atrial appendage occlusion (LAAO) is an option for AF with high bleeding risk or relative/absolute contraindication for long-term anticoagulation (Class IIbB) or for those with AF undergoing cardiac surgery (Class IIbC).11 Evidence of the LAAO role in CHD is centred on ASDs, where it has proven to be safe and feasible when implanted before or at the time of ASD closure.97 In other conditions with very high thromboembolic risk, such as patients with mechanical prostheses, anticoagulation cannot be stopped despite LAAO, although it could be considered as a means to reduce embolic risk.98
Future Directions
The high and increasing prevalence of AF in the expanding CHD population requires rapid advances in knowledge. Research in the underlying mechanisms that differ from the general population should be pursued, as a better understanding has the potential to translate into improved therapies and better outcomes for patients. Strategies for diagnosis and correction of risk factors and identification of particularly vulnerable patients are necessary. The use of prophylactic surgical or percutaneous ablation concomitant to defect correction needs to be explored. Also, appropriate timing, techniques and targets for catheter and surgical ablation need to be defined. Studies comparing anticoagulation strategies in the CHD population are warranted. The role of NOACs for patients with Fontan circulation and cyanotic conditions needs to be further explored to establish evidence-based recommendations.
Overcoming these and future challenges will require multicentre or network collaboration so large cohorts of CHD patients can be studied.