Psoriasis is a chronic inflammatory skin disorder, and one of the most common skin diseases that presents in everyday dermatology practice. It is characterised by overgrowth of keratinocytes in the skin tissue, with complex underlying inflammatory processes playing a role in its pathogenesis.1 An extensive inflammatory response in different body systems aggravated by a dysregulated immune system is thought to play a pivotal role in its pathogenesis.2–4 The correlation between these complicated immune and non-immune responses have been thoroughly investigated to establish an appropriate management strategy for the disease and to set up a foundation for its multifactorial association with other systemic comorbidities, most importantly cardiac diseases.5
According to the General Practice Research Database (GPRD) there is a strong suggestion that there is an increased independent association between cardiovascular disease (CVD) and severe psoriasis, particularly in younger patients.6 This was further confirmed in a study performed by Ludwig et al., who suggested that psoriasis could act as an independent risk factor for composite cardiovascular outcomes.7 However, this suggestion was soon opposed by another GPRD database study by Parisi et al. who abandoned the hypothesis of the relationship between the two entities even after the adjustment for traditional risk factors.8
Nevertheless, the prevalence of CVDs as a leading cause for higher mortality rates among patients with psoriasis compared to other causes such as infection or end-stage disease.2,3,9 Hypertension, diabetes, hyperlipidaemia and non-alcohol fatty liver disease are all modifiable risk factors in CVDs and they have been reported in psoriasis with a parallel rise in accordance with disease severity.2,3,10,11 This is of paramount importance among all systemic comorbidities caused by psoriasis, due to its direct effect on life expectancy, especially in advanced cases.2 The disease is not only confined to the skin tissue. It has various systematic, cellular, immune and non-immune mechanisms referred to as psoriasis march.12 This concept was first introduced in 2011 as a notion that severe psoriatic inflammation may lead to insulin resistance and vascular dysfunction preceding acute MI and stroke development.13 However, there is still an independent association between psoriasis and CVD.
Epidemiology
Psoriasis is a chronic inflammatory multisystemic skin condition, primarily affecting skin and joints.14 It is characterised by scaly skin lesions in the form of patches, plaques or pustules with episodes of relapse and remission. The prevalence of psoriasis is approximately 2% worldwide, with more than 50% of the cases presenting in the first three decades of life.15 There are two common ages for its predominance: the initial presentation is usually experienced during the second decade of life while the second presentation often occurs in the patient’s fifties.16 The prevalence of psoriasis varies according to geographical location, with higher rates among Caucasians when compared with other ethnicities.10 It is also argued that countries located away from the equator have shown a higher prevalence of the disease when compared to warmer climates where the reported cases are relatively lower.17 This variation is thought to be related to various degrees of genetic and environmental factors.10 Nevertheless, cardiac diseases are also more common in these geographical locations and that could be attributed to similar risk factors.
The life expectancy of people with psoriasis was reported to be nearly 5 years lower compared to control groups, with cardiovascular problems being the main cause of death.9 The disease also has a significant impact on physical and mental well-being, with a subsequent impact on heart disease as a result.18,19 The majority of deaths for people with psoriasis are related to cardiovascular or cerebrovascular morbidities, such as MI or stroke, and not solely to renal diseases or infection as previously thought.9 There is evidence of an increased risk of major cardiovascular events in patients with psoriasis and it has a direct and linear relationship with disease severity.20 Ahlehoff et al. noted that the risk of cardiovascular-related death was higher with severe psoriasis (RR 1.39; 95% CI [1.11–1.74]) rather than with a mild form of psoriasis (RR 1.03; 95% CI [0.86–1.25]).21
Risk Factors in Psoriasis and CVD
Genetic
The association between psoriasis and CVD has led researchers to further investigate the links between the two in the hope of discovering genetic loci shared between them. However, to date, there is no established genetic association between psoriasis and CVD shown in the Genome-wide Association Studies. Observational studies have shown that psoriasis patients have high levels of homocysteine, resulting from demethylation of DNA. It was found that polymorphism of methylenetetrahydrofolate reductase can lead to DNA methylation, and several studies have demonstrated that homocysteine is an independent risk factor in CVD.22 The caspase recruitment domain-containing protein 14 (CARD14) gene has been investigated for its mutation and the link with psoriasis. CARD14 is mainly expressed in dermal keratinocytes and some unidentified dermal cells.23 A study by Harden et al. highlighted the expression of CARD14 in aortic endothelial cells, which might indicate a relation between CVD and psoriasis; this is an area that requires further research.24
Environmental
Obesity has been thought to be a key contributor in the development of both diseases. A strong link between high BMI and the development of both diseases has been explained in a robust systematic review which demonstrated that the incidence and prevalence of obesity are higher in patients with psoriasis and it is independently associated with CVD.25 Furthermore, obesity is linked to the metabolic syndrome, which induces a systemic inflammation caused by five pathological mechanisms: abdominal obesity, impaired glucose tolerance, hypertriglyceridaemia, hypertension and low levels of HDL cholesterol. A meta-analysis conducted by Choudhary et el. demonstrated that 30.3% of patients with psoriasis had metabolic syndrome.26 Similarly, Mottillo et al. reported that the metabolic syndrome is associated with a twofold increase in cardiovascular outcomes and a 1.5-fold increase in all-cause mortality.27 Visceral adipose tissue triggers the release of adipokines, which in turn lead to the development of insulin resistance and endothelial cell damage. This cascade of events prompts the formation of atherosclerotic plaques which enhances the process of CVD.28
Psychological
Stress is another factor implicated in the aetiology of both diseases, with its negative sequelae in leukocyte catalysis and surge of inflammatory factors in the tissue areas. It was thought that patients with higher levels of stress can have severe psoriatic flare ups. However, recent studies have not revealed a strong link between perceived stress and the severity degree of psoriasis.29
Social
Smoking is a strongly recognised risk factor for CVD. It is also implicated in the disease process of psoriasis. The disease prevalence is higher among smokers and continues to rise despite smoking cessation. It has been demonstrated that patients who smoke more than 20 cigarettes per day are associated with a twofold risk of severe psoriasis with more cases reported of pustular psoriasis.30,31 Moreover, the relationship between alcohol and CVD is well established, whereas it is more complex with psoriasis. A higher consumption of alcohol has been seen in patients with severe psoriasis and studies have confirmed a rise in CVD in these subgroups.32,33
The Pathogenic Connection Between Psoriasis and Vascular Disease
The precise correlation between psoriasis and CVD is not yet fully understood. The hypothesis of the impact of deep inflammatory processes at cellular level has been postulated.4 Inflammatory mediators with various cytokines have been identified as playing a major role in psoriasis, including interleukins (IL)-1,4,6,8 and 12 and tumour necrosis factor-α (TNF-α).34
Atherosclerosis
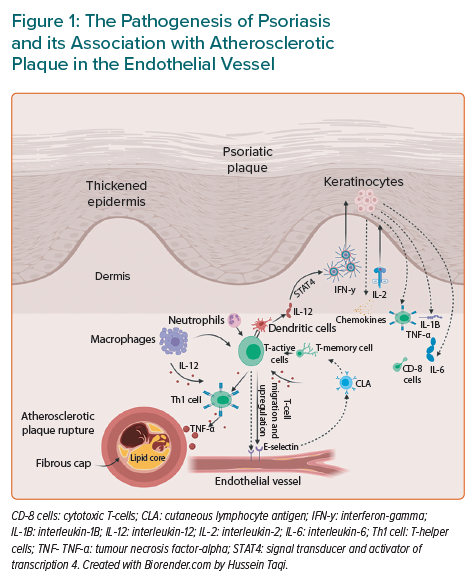
Atherosclerosis is a progressive disease caused by the deposition of lipid with a superimposed fibrous cap. This often occurs over decades, eventually leading to cardiovascular and cerebrovascular diseases, such as MI and stroke.35 Studies have also found that the underlying inflammation plays an essential role in the progression of atheroma and the eventual rapture of the plaque.36 At cellular level, atherosclerosis is associated with inflammatory cytokines, such as TNF-α and IL-1, which overlap with the same markers implicated in psoriasis (Figure 1).37
This process initially starts with T-helper cells 1 (Th1), which are a subdivision of active T-cells and the main active T-cells in psoriasis followed by the activation of macrophages, neutrophils and CD8 cytotoxic T-lymphocyte.38,39 In psoriatic plaque, Th1 cytokines, interferon-y (INF-y), IL-2, and TNF-α catalyse keratinocytes and stimulate the production of inflammatory cytokines (TNF-α, IL-1β and IL-6) and chemokines ligands 8, 9, 10, 11 and 20.40 Following this, the migrated T-cells to the dermis require upregulation of adhesive molecules and once E-selectin is upregulated in the dermal microvasculature of inflamed cutaneous tissue, it acts as a ligand for the cutaneous lymphocyte antigen in the memory T-cells.
Consequently, the binding of T-cells to the adhesion molecules will further enhance the migration of more T-cells into the dermis and hence provoke more inflammation. Simultaneously, the raised levels of circulating Th1 cytokines such as TNF-α also lead to endothelial dysfunction and extravasation of T-cells into the site of atherosclerotic plaque.41 The activated dendritic cells produce IL-12, which will later activate transcription factor signal transducer and activator of transcription 4 and subsequently trigger the release of large amounts of INF-y, leading to further Th1 differentiation. The activation of Th1 enhanced by IL-12 is well recognised in psoriasis and atherosclerosis, therefore, there would be assumptions that downstream release of cytokines are shared in the pathogenesis of the two conditions.35
More recent studies have shown that patients with psoriasis have an increased non-calcified lipid-rich atherosclerotic coronary plaque burden (NCB). This NCB has been reported to be mediated by immunological abnormalities and impaired HDL function.42 In fact, psoriasis patients have significantly higher levels of oxidation-modified lipids (OMLs), including oxidised LDL (oxLDL), HDL (oxHDL) and lipoprotein (a) – oxLp(a) – which promote a proatherogenic lipoprotein profile and an impaired HDL-cholesterol efflux capacity (CEC).42 Furthermore, oxLDL initiates a cascade of biochemical reactions leading to endothelial cell dysfunction and atherosclerotic plaque formation.43 Additionally, in vitro experiments have shown low doses of oxLDL to be sufficient to activate macrophages and mast cells to synergistically increase monocyte-endothelium adhesion, which contributes to endothelial dysfunction and early atherogenesis.44
Myeloid-derived suppressor cells (MDSCs) are a heterogeneous group of progenitor and immature myeloid cells, which are produced in a variety of conditions such as cancer, infectious diseases and autoimmune disorders. Studies have demonstrated that the population of MDSCs has expanded in psoriasis patients, where cytokines including IL-23, IL-1β and CCL4 have been produced.45,46 It is now appreciated that myeloid dendritic cells are key cells in the pathogenesis of psoriasis. Inflammatory myeloid cells release IL-23 and IL-12 to activate IL-17-producing T-cells, Th1 cells and Th22 cells to where psoriatic cytokines IL-17, IFN-γ, TNF and IL-22 have been generated. These cytokines express effects on keratinocytes to amplify psoriatic inflammation.47 Myeloid cells expressing 6-sulfo-LacNAc (Slan) have also been thought to be inflammatory DC precursors in psoriasis, driving strong T-helper cells (Th17) and Th1 responses.
Platelets are also immune cells that trigger and regulate immune and inflammatory processes and their dysfunction is deeply involved in the pathogenesis of psoriasis.48 Liu et al. demonstrated that platelets from patients with psoriasis showed a significantly increased percentage of platelet aggregation, which is positively correlated with disease severity. Additionally, activated platelets could stimulate an inflammatory environment by releasing biologically active molecules in some skin disorders, such as psoriasis, systemic lupus erythematous and multiple sclerosis.49 Cytokines released by activated platelets are reported to play a key role in psoriasis. Platelets are the major source of IL-1β, and an increased IL-1 level is involved in the development and progression of inflammation in psoriasis. Serotonin derived from platelet-dense granules is also expressed in psoriatic skin to modulate the immune response.50
Psoriasis and Coronary Microvascular Dysfunction
The chronic exposure to systemic inflammation in patients with severe psoriasis can result in coronary microvascular dysfunction (CMD). This may lead to the impairment of the coronary arteries’ ability to augment coronary blood flow (vasodilatory abnormality) and/or in a reduction in coronary blood flow (coronary microvascular spasm).51 Piaserico et al. showed that CMD was associated with severe psoriasis in 15% of the 153 patients in their study (n=23; OR 3.1, p=0.03).52 Similarly, the prevalence of CMD has been assessed in psoriasis patients in a cohort study by Weber et al. to quantify myocardial perfusion and myocardial flow reserve (MFR) using positron emission tomography imaging. It was reported that the prevalence of CMD (defined as MFR <2) was 61.3% in patients with psoriatic disease, compared to 38.4% in a matched control population (p=0.004). Additionally, psoriasis patients had a more drastic reduction in adjusted MFR (2.3 ± 0.81 versus 1.92 ± 0.65, p=0.001, respectively).53
Endothelial dysfunction is a key mechanism for obstructive and non-obstructive forms of coronary artery disease. It is referred to the imbalance between the release of vasoprotective vasorelaxant mediators, such as nitric oxide (NO), prostacyclin (PGI2), endothelium-derived hyperpolarising factors, and pathological vasoconstricting substances, such as endothelin-1 (ET-1), superoxide, hydrogen peroxide and thromboxanes.54 To evaluate endothelium-independent microvascular functional status, the coronary flow reserve (CFR) can be measured, which is defined by the rate of coronary blood flow at hyperaemia compared to baseline. The cut-off for an abnormal CFR is ≤2.5 or 2.0, depending on the technique that is being used.55
In 2012, Osto et al. reported that in patients with psoriasis, CFR was lower than in controls (3.2 ± 0.9 versus 3.7 ± 0.7, p=0.02). The CFR was abnormal (≤2.5) in 12 patients (22% versus 0% controls, p<0.0001). Moreover, in patients with CFR ≤2.5, the psoriasis area severity index (PASI) score, a clinical score for psoriasis severity was higher (11 ± 6 versus 7 ± 3, p=0.006) compared to patients with CFR >2.5. At multivariable analysis, PASI remained the only determinant of CFR ≤2.5 (p=0.02).56 Amplified adrenoreceptor-mediated vasoconstrictor responsiveness is another factor contributing to vascular dysfunction in pathological conditions characterised by systemic inflammation such as psoriasis.57 Increased arterial stiffness has also been reported in patients with psoriasis.58
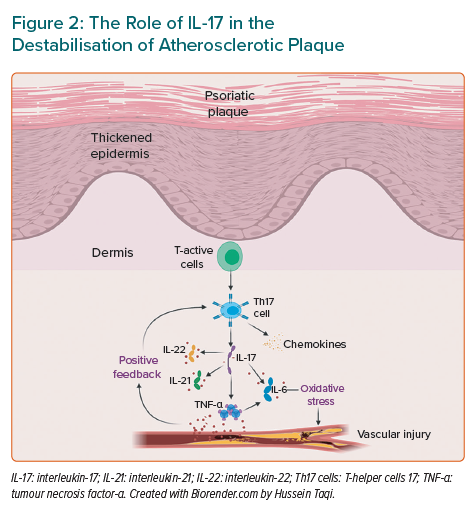
Interleukin-17
Another cytokine which is a key element in the pathogenesis of both conditions is IL-17 (Figure 2). This is a pro-inflammatory cytokine released by Th17, which similarly produces IL-6, IL-21, IL-22 and TNF-α. All these factors are involved in inflammatory diseases such as psoriasis and atherosclerosis. Th17 plays a crucial role in the atherosclerotic process with larger levels measured in patients with CVDs.59 Its release into endothelial and vascular smooth muscle cells will render arteries vulnerable to a pro-inflammatory feedback loop.60 Once there is an endothelial injury, there is a release of cytokines that stimulates more Th17 which in turn fuels its release in positive feedback. This process consequently potentiates the release of further pro-inflammatory cytokines, such as TNF-α, adhesion molecules and chemokines. Eventually, TNF-α and IL-17 work collaboratively to upregulate other key cytokines, such as IL-6. It has been demonstrated that these inflammatory cascades enhance local oxidative stress, which causes the plaque to be less stable. Th1 cells are thought to have a role in the pathophysiology of ischaemic heart diseases in psoriasis.61
Nitric Oxide
Various studies have suggested that NO is released in many inflammatory diseases with psoriasis being one of them.62 The synthesis of induced NO is shown to be correlated with an increased release of NO-forming enzymes in psoriasis. Similarly, the involvement of NO in the function of endothelial cells suggests that it is implicated in the pathogenesis of the disease.63 NO is highly involved in the activation of IL-1β, cyclo-oxygenase and TNF-α, which are major contributors in psoriasis and the inflammatory processes of atherosclerosis. This hypothesis is further enhanced due to the abundance of NO in a diseased tissue compared to normal samples.
NO also plays an important role in the pathology of atherosclerosis by affecting the arterial wall through catalysing inflammatory cells such as macrophages and leukocytes. NO affects the resting vascular tone, permeability and alters the metabolism that is associated with the inflammation in psoriasis patients. This led to the assumption that induction of NO synthesis by the high level of enzymes in psoriasis and its action in cells outside the skin, such as endothelial cells, could alter vascular function and lead to CVD.64
Oxidative Stress
Oxidative stress tends to have a key element in the development of CVD in patients with psoriasis. It is thought that this phenomenon participates in the process of atherosclerosis by the effect of oxygen metabolites, which modifies oxidatively LDL cholesterol and the antioxidant capacity is consequently overwhelmed. The resulting oxidative stress leads to oxidative damage to the lipids and proteins.65 This discrepancy between oxidant and antioxidant factors is involved in the pathogenesis of the accelerated atherosclerosis in psoriasis.66
Angiotensin-converting Enzyme, Renin and Endothelin-1
High levels of angiotensin-converting enzyme levels, renin and ET-1 in psoriasis are associated with an increased risk of hypertension. It is believed that the adipose tissue in psoriatic patients acts as a source of angiotensinogen that is converted to angiotensin II.11 It has been observed that angiotensin II has a dual function in this condition; it retains salt in the kidneys and participates in the proliferation of T-cells, which consequently influences the inflammatory process of atherosclerosis. Additionally, ET-1 can cause accelerated hypertension by affecting the vascular endothelium which acts as an intermediate border in the circulation and its levels are higher with more severe cases.67
Pharmacotherapy in Psoriasis and its Correlation with Cardiovascular Diseases
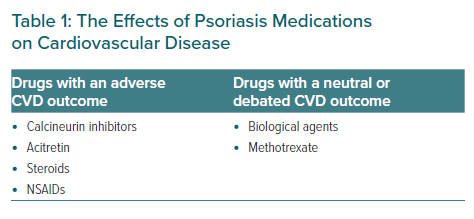
The pharmacotherapy used in psoriasis has a dual effect on patients’ cardiovascular risk profile (Figure 2). Ciclosporin is an effective treatment in psoriasis but it has been strongly linked to the worsening of hypertension. The underlying reason for the predominance of essential hypertension due to systemic ciclosporin is primarily due to the development of chronic kidney disease.68 Ciclosporin also alters vascular endothelial function by suppressing vasodilators such as prostacyclin and NO, whereas vasoconstrictors are being catalysed by increasing levels of endothelin. The cumulative effects of vasoconstriction, sodium retention and the reduction of glomerular filtration collectively act as a precursor for worsening hypertension. It has been argued that calcineurin inhibitors enhance the action of the thiazide-sensitive sodium chloride co-transport through a direct effect on the tissue kinase activity resulting in high rates of hypertension.69 For that reason, it is strongly advised to use ciclosporin only for a short period and there should be an intention to substitute it with another systemic agent once the flare up has improved.70
Steroids have an effective role in the treatment of psoriasis but they are implicated in heart diseases. They can potentially cause hypertension by various mechanisms which include increased systemic vascular resistance, extracellular volume and cardiac contractility. They also cause sodium retention, hypokalaemia and hypertension by altering the blood pressure regulatory system.71 Similarly, the non-steroidal anti-inflammatory drugs have similar consequences mainly by causing salt and water retention, aggravating peripheral vascular resistance and the activation of the renin-angiotensin-aldosterone system.72 Acitretin, which is a vitamin A analogue used in the treatment of psoriasis, has unfavourable effects by increasing the levels of serum triglycerides and LDL cholesterol and reducing HDL levels in the blood.73
A growing body of evidence pointing mainly towards the shared pathophysiology of psoriasis and CVD has raised an important question about the possibilities that treatment of the cutaneous disease can reduce the development of cardiovascular risk (Table 1). This hypothesis mainly suggests that inflammatory cascades in psoriasis, specifically those related to atherosclerosis, could be prevented by certain medications and reduce major adverse cardiovascular events (MACE) as a consequence.
For example, methotrexate is one of the oldest systemic agents used in the treatment of psoriasis and was thought to have new promises in reducing vascular diseases in patients who suffer from psoriasis if matched to the control group in some studies.34 However, the CIRT trial showed that low-dose methotrexate did not reduce IL-1β, IL-6, C-reactive protein (CRP) or cardiovascular events compared with placebo among patients with established coronary artery disease and either diabetes or metabolic syndrome or both.74
The biological agents that are commonly used in moderate-to-severe psoriasis have shown neutral outcomes in reducing MACE in the short term.12 Their effects have been questioned despite their direct action on the same cytokines involved in the pathogenesis of psoriasis and atherosclerosis such as TNF-α, IL-12, 23 and 17. Furthermore, the link between biological medications and the development of MACE is still unclear as shown in multiple meta-analyses, therefore, more robust long-term studies are required in this field.75,76
In 2019, a 5-year study showed that tumour necrosis factor inhibitor (TNFi) can significantly reduce the risk of MACE, which is cumulative and can reach up to 11% reduction in cardiovascular events in 2 years.77 In the VIP trials, adalimumab was compared with phototherapy and showed a reduction in inflammatory markers such as glycoprotein acetylation in comparison with phototherapy. However, adalimumab did not have an effect on vascular inflammation.78 In contrast, when ustekinumab was compared with placebo in VIP trials, patients assigned to ustekinumab had a -6.58% (95% CI -13.64–0.47%) reduction in aortic vascular inflammation (AVI) at week 12 compared with baseline, whereas patients assigned to placebo had a 12.07% (95% CI 3.26–20.88%) increase in AVI during the same time period.79
The effect of TNF-α on major cardiovascular events was investigated in a meta-analysis and a net benefit in reducing adverse events was reported compared to methotrexate, warranting further randomised controlled trials to validate these findings.80 Notably, elevated levels of TNF-α and soluble TNF-α receptors were found in the severe psoriatic skin lesions, which likewise reflect the same findings in heart failure.81 TNF-α activation can result in different consequences: the development of atherosclerosis; deterioration in the heart function; and remodelling of the vascular endothelium. Anti-TNF-α can minimise CRP, vascular endothelial growth factor (VEGF) and an array of chemotactic co-factors – epithelial cell adhesion molecule-1, IL-8, E-selectin and monocyte chemoattractant protein-1 – in addition to the Th17 levels in the peripheral blood of patients with psoriasis.82
Although there are multiple studies that contributed to the body of evidence of effectiveness of TNFi in reducing systemic inflammation, it is still an area of debate with regards to its effectiveness in reducing CVD. Boehncke et al. revealed that patients who have been commenced on TNFi not only showed improvement in PASI score, but they also demonstrated a decline in CVD biomarkers (CRP, VEGF and resistin levels) after 6 months of therapy.78 Additional studies also endorsed the improvement of endothelial function and insulin sensitivity after starting etanercept (a TNFi) in severe psoriatic patients.83,84 The same authors also approved the reduction in the serum retinol binding protein 4 (RBP4) in the treated psoriatic patients who received TNFi.85 RBP4 has a strong link on the other side with subclinical atherosclerosis.86 Another clinical trial, which is based on echocardiographic assessment of the heart function for 18 patients receiving TNFi showed an improvement in the cardiac systolic function.87 Similarly, a small study consisting of 44 patients showed an improvement in the right ventricular systolic function in 33 patients treated with TNFi.88 On the contrary, a larger study of 1,500 patients with symptomatic heart failure treated with etanercept showed no improvement in mortality or hospitalisation.89 Despite the wide difference and interpretation of TNFi’s impact on CVD profile, which is mainly due to the different methodological approaches, there are some data that revealed a positive role for TNFi in the reduction of CVD, which could prompt researchers to investigate its effectiveness in future.12
Conclusion
Various studies have explored the link between cardiovascular complications and psoriasis. Different aspects have been investigated, such as genetic, environmental and social risk factors. The possible pathogenic and pharmacological connection between the two diseases has also been investigated. It can be concluded that a relation between CVD and psoriasis is possible, but there are other avenues that need to be explored and more in-depth research on a wider scale is required.
It is imperative that GPs recognise early signs of cardiovascular complications of psoriasis and seek advice from cardiology specialists when indicated. Formulating an appropriate follow-up plan and encouraging joint management would improve patient outcome through early detection and management of those complications.