Arrhythmias are common in all age groups, and becoming more prevalent with increasing age.
In young people, most cases reflect the presence of congenital anomalies of the structure or function of the conduction system of the heart. These affect approximately 1% of the general population and, although seen in patients with renal conditions, they have no important association with chronic kidney disease (CKD).
Acquired conditions of the atrial and ventricular myocardium accumulate with age and cause atrial and ventricular tachyarrhythmias and bradyarrhythmias. AF is the most common sustained arrhythmia by far; it increases sharply with age and affects 1.5% of the general population at age 55–59 years and 27% at age >85 years.1 Sustained and recurrent ventricular arrhythmias are less common, but are important as sudden death is often due to ventricular tachyarrhythmia. Complete atrioventricular block and other forms of bradyarrhythmia are common and increase sharply with age.
CKD is even more prevalent than sustained arrhythmia and is associated with an excess of acquired arrhythmia of multiple types, and AF in particular.2 Sudden death is also more common in CKD and accounts for around one-quarter of deaths in dialysis patients.3
Rigorous monitoring can detect a higher incidence of arrhythmia than is evident clinically. Physical or electrocardiographic examination performed in response to symptoms catches a minority of events. In the ARIC study, a 2-week cardiac monitor recorded a high prevalence of non-sustained ventricular tachycardia (30.2%) and AF (7.4%) in patients with CKD, while ectopy was present in >90% of patients.4
The most intensive monitoring is that provided by an implanted device. Rautavaara et al. studied 71 dialysis patients who were asymptomatic for arrhythmia; in a follow-up of 34 months, they detected AF in 51% of patients, significant bradycardia in 24% and ventricular tachycardia in 23%.5
Mechanisms of Arrhythmia in Renal Failure
Common Causes
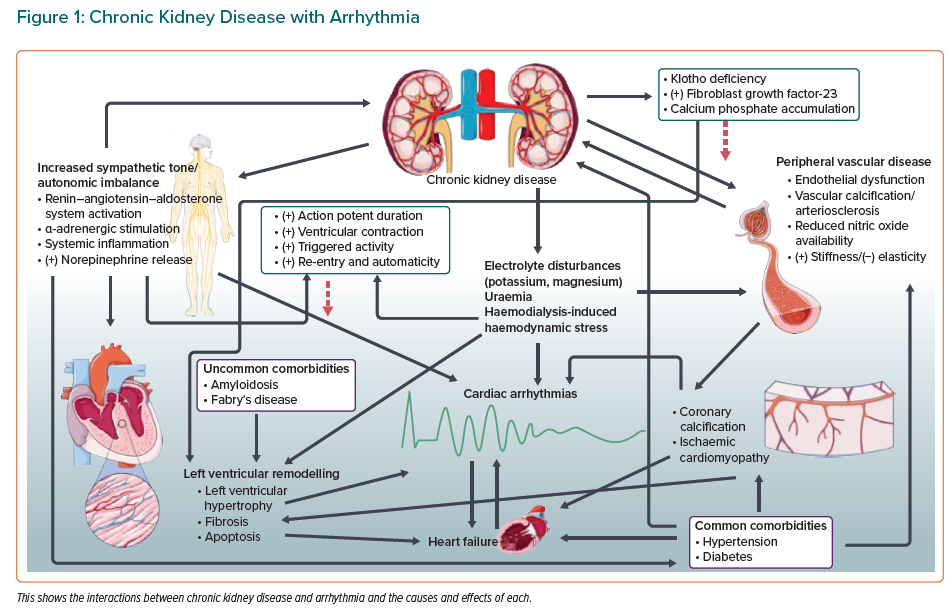
Renal and cardiac tissue share a vulnerability to damage from conditions that are common throughout the world (Figure 1).
Diabetes and hypertension each account for a large proportion of arrhythmias in the general population, particularly AF. Both conditions are also responsible for a large proportion of cases of end-stage renal failure.
In both cases, CKD and AF are usually late effects of the underlying condition, but that underlying condition commonly goes undiagnosed until the consequences bring it to light.
Uncommon Causes
A number of uncommon and rare syndromes are associated with both arrhythmia and CKD. Despite this rarity, they are important because prompt recognition may permit life-extending specific treatment.
Fabry’s disease is an X-linked lysosomal storage disorder characterised by an accumulation of glycosphingolipids resulting from a deficiency in the enzyme α-galactosidase A.6 There is systemic deposition of glycosphingolipids (particularly globotriaosylceramide) including in the cells of the blood vessels, kidneys and heart.7 Cardiac infiltration subsequently results in left ventricular hypertrophy (LVH) secondary to myocardial fibrosis, while renal involvement leads to CKD. Unsurprisingly, cardiac and renal involvement is common in Fabry’s disease; a small study of patients (average age 25 years) with Fabry’s disease found that 42% of patients already had CKD at the point of diagnosis and 33.3% had LVH, suggesting early simultaneous organ involvement.8
Arrhythmias in this population are not rare. An observational study has reported a prevalence of 13.3% in a cohort with Fabry’s disease, although it could be higher as the risk worsens with age.8,9 Atrial, ventricular and bradyarrhythmias have been confirmed.9,10
Myocardial fibrosis is an important substrate for arrhythmias; it is associated with a significantly higher risk of arrhythmias comparatively to patients without fibrosis.11 The risk of arrhythmias may also be compounded by CKD, which is a pro-arrhythmic clinical state in its own right.12 It is therefore unsurprising that the cause of mortality in this group of patients is sudden cardiac death (SCD).13 This may be by ventricular tachyarrhythmias or by bradyarrhythmias.10,13
Amyloidosis, like Fabry’s disease, involves infiltration of both the myocardium and kidneys. There is aggregation and deposition of abnormal protein – amyloid – in the healthy extracellular tissues resulting in organ damage.14 Although there are numerous types, the most common in the western world is primary amyloidosis; here, immunoglobulin light-chain proteins are deposited in the affected organs.14,15
Renal impairment is a feature of this illness, with associated poor outcomes, even when compared to patients with CKD from other aetiologies.14 Nephrotic syndrome ensues progression to end-stage renal failure requiring renal replacement therapy and/or renal transplant. Despite these interventions, outcomes are unfavourable compared to the general renal failure population.14
There is cardiac involvement not only directly through the disease itself but also from renal replacement therapy; progressive haemodialysis can become inefficient at filtration, resulting in β2-microglobulin deposition from the uraemia.15
Cardiac involvement in amyloidosis results in a restrictive cardiomyopathy leading to diastolic left ventricular (LV) dysfunction. Arrhythmias are common in this subgroup of patients, including AF, ventricular tachyarrhythmias and conduction abnormalities.16 Evidence suggests these patients do not tolerate arrhythmias well due to the poor compliance of the cardiac muscle, which compounds the abnormal filling and ejection of blood.16
Arrhythmia management is also difficult because the tissue has abnormal properties following amyloid infiltration. Traditional pharmacological therapy, including β-blockers, calcium channel blockers and digoxin are poorly tolerated because of the altered haemodynamics, while amiodarone, although it maintains sinus rhythm, is associated with significant side effects.16
Catheter ablation, the treatment of choice in many arrhythmias, also is associated with variable outcomes; a small study found a high 1-year arrhythmia recurrence rate following catheter ablation in patients with amyloidosis compared to a similar set of patients without the condition.17 Although cardiac failure is the most common cause of mortality in patients with amyloidosis, SCD remains a concern.18 SCD can include ventricular arrhythmias but also pulseless electrical activity with electromechanical asynchrony.
Chronic Kidney Disease, Mineral Bone Disorders and Anaemia
A key feature of CKD is the development of CKD mineral bone disorders. This syndrome is characterised by altered calcium, phosphate, parathyroid hormone, vitamin D and fibroblast growth factor-23 (FGF-23) homeostasis; vascular or soft tissue calcification; and an abnormal bone structure and/or turnover.19 This has a number of effects on the cardiovascular system.
Calcium is a crucial component of myocyte depolarisation and cardiac contractility, while phosphate is central to adenosine triphosphate, the energy-carrying molecule that cells rely upon.
FGF-23 regulates circulating phosphate and vitamin D levels and is associated with poor outcomes. In a study of 795 patients, FGF-23 was strongly associated with LV hypertrophy and increased LV mass index; higher LV mass index is associated with SCD.20,21 It also may play a role in the calcification of coronary and peripheral arterial vessels which, in turn, lead to cardiovascular events, thereby exacerbating the risk of SCD.22,23
Vitamin D deficiency in CKD has also been found to be associated with cardiac dysfunction. A small prospective control study of 25 patients found that treatment with calcitriol, the active form of vitamin D, markedly reduced LV hypertrophy, resulting in an improvement in LV function.24 This indicates that vitamin D plays a significant role in maintaining cardiovascular health in CKD. The authors discovered an association between calcitriol and lower levels of circulating parathyroid hormone and angiotensin II; they proposed that vitamin D may have lowered the level of these neurohormones, which affect LV mass through direct or indirect mechanisms.
Anaemia is common in people with CKD owing to erythropoeitin deficiency and is associated with excess mortality. A large retrospective study suggested that haemoglobin <6.52 mmol/l was associated with a mortality risk (HR 5.27) and anaemia was independently associated with mortality and cardiovascular events.25
Anaemia in CKD has been associated with LVH, which is an established variable for poor cardiovascular outcomes, and there is evidence suggesting that correction of anaemia results in LVH regression.26
However, randomised controlled trials have demonstrated no cardiovascular benefit and, in some cases, worse outcome from correction of anaemia with erythropoeitin.27,28 This is probably because the benefits from erythropoeitin were negated by the adverse effects from this hormone; promoting red cell production can increase blood viscosity (therefore increase the risk of thrombosis) while attenuating hypertension.
Ischaemia
Patients with CKD develop ischaemic heart disease at a greater rate than the general population.
Although effective lipid-lowering therapy has been available for decades, re-entry around scarring from previous MI is the leading cause of sustained ventricular tachycardia, while ventricular dysfunction from chronic ischaemia is a major cause of heart failure. Between them, these account for a large proportion of SCD.
Atrial arrhythmia is not so strongly linked to ischaemia. Typically, atrial flutter is more common in those with ischaemic heart disease than in age-matched controls, while AF occurs at similar rates in both groups.
Electrolytes
The kidney regulates the excretion or retention of electrolytes and products of metabolism in a continuous manner; dialysis is intermittent, often occurring at intervals of several days. The discontinuous nature of the dialysis process inevitably leads to fluctuations in the levels of any variable that would normally be kept constant by the kidney.
The extent of fluctuation is not itself constant: potassium can accumulate unexpectedly due to changes in diet and variation in the severity of renal dysfunction. During haemodialysis, changes in serum potassium concentration exceeding 1 mmol/l commonly occur in a period of a few hours.29
Trans-membrane ionic gradients drive the electrophysiology of excitable tissues, including the myocardium. Potassium and sodium are involved, but the process is particularly vulnerable to abnormalities in potassium concentration because the resting membrane potential of excitable cells is identical to that of the equilibrium potential of potassium.30
Hyperkalaemia is a common feature of renal failure. It produces characteristic abnormalities of the ECG, including peaked T-waves, P-wave flattening and broadening of the QRS duration.
At higher levels of hyperkalaemia, conduction block, bradyarrhythmias, asystole and ventricular arrhythmias can occur. Physiologically, these are related to the raised extracellular potassium concentration; this shortens the myocyte action potential duration (APD) and slows conduction velocity which, in turn, affects myocardial refractoriness.30
At high extracellular potassium levels, there is risk of heart block and asystole as the conduction velocity slows, with the shortened APD causing widespread myocardial refractoriness. Ventricular arrhythmias in hyperkalaemia are thought to be re-entrant circuits. It is hypothesised that there is APD discordance in localised regions of the heart with progressive hyperkalaemia. This generates areas of localised block and potentially re-entry.30
Hyperkalaemia in CKD can occur catastrophically as part of a constellation of mutually reinforcing processes featuring bradycardia, renal failure, atrioventricular block, shock and hyperkalaemia (BRASH) itself. This cycle can be triggered by the synergy between bradycardia and hyperkalaemia, each of them easily provoked pharmaceutically.31
For example, β-blockers and calcium channel blockers commonly used for controlling arrhythmias can cause bradycardia, which in patients with renal impairment can trigger BRASH syndrome; the risk is believed to be greatest in elderly patients being treated for AF.31 Once established, this reinforcing sequence can progress to death unless interrupted by supportive care to correct the bradycardia and the hyperkalaemia; temporary pacing may be required as part of this basic support.
Neuropathy
The autonomic nervous system regulates the heart rate in sinus rhythm and in a less precise manner in AF. Feedback mechanisms mediated by this system appear to have a role in stabilising the electrophysiology of the myocardium, or at least the capacity to destabilise it when the system malfunctions.
A role for autonomic dysfunction in the genesis of AF has been hypothesised, and partial cardiac denervation has been proposed as part of the reason for rhythm stabilisation after AF ablation.
Autonomic neuropathy is a common consequence of CKD and of the diabetes that often underlies it.32 It is reasonable to hypothesise that this neuropathy might contribute to the arrhythmias seen in patients with CKD.
There is evidence suggestive of sympathetic overactivity in CKD. This has numerous adverse effects on the renal-cardiovascular systems. Sympathetic overactivity can exacerbate hypertension, which can sequentially worsen the renal impairment; hypertension contributes to interstitial fibrosis and glomerulosclerosis.33 Simultaneously, sympathetic overactivity can cause LVH, directly or indirectly, and it has a known association with cardiac arrhythmias.34
There is strong evidence to suggest patients with CKD are more likely to develop AF.35 This association was suggested to be causal in a canine model via an autonomic cross link. In this study of 28 dogs, renal sympathetic nerve (RSN) activation mediated pro-fibrillatory effects in the pulmonary veins and atria; RSN activation increased AF inducibility.36
Although this is yet to be proven in human studies, it has a theoretical application. Patients with CKD have documented increased RSN activation and RSN denervation has been shown to be an effective therapy for treating AF.37 A better understanding of the mechanism of RSN hyperactivity may present significant therapeutic strategies for arrhythmias.
Inflammation
The pathogenesis of AF in CKD could be linked on a molecular level. A recent study has suggested a role for the NLRP-3 inflammasome in the pathophysiology of AF.38
The NLRP-3 inflammasome is a component of the innate immune system and has been shown to act on cardiomyocytes and atrial fibroblasts.39 Activated cardiomyocytes and fibroblasts can secrete inflammatory cytokines, recruit macrophages and other inflammatory cells, and induce atrial fibrosis.38
Atrial fibrosis is an arrhythmogenic substrate as it disrupts the normal cellular architecture and therefore impairs normal conduction. This increases conduction heterogeneity, producing re-entrant mechanisms to sustain AF.38,39
The significant role of NLRP3 inflammasome in renal injury is recognised and a recent mouse model study (sham-operated versus CKD mice) demonstrated a significantly elevated level of NLRP3 in the cardiac tissue of the CKD mice.40,41 Therefore, it is suggested that CKD upregulates NLRP3 in cardiomyocytes and promotes arrhythmias.
Renin–Aldosterone–Angiotensin System
Patients with CKD have been shown to have inappropriately high renin-angiotensin-aldosterone system (RAAS) activity.42 Molecules within the RAAS system have been implicated in inflammation, atrial enlargement and atrial fibrosis.43–45
First, RAAS has been shown to upregulate inflammatory cytokines such as IL-6 and increase cell adhesion.46,47 Second, in an animal study, increased angiotensin-converting enzyme (ACE) expression has been shown to increase atrial size, leading to increased atrial arrhythmias, and angiotensin II, the main active molecule of RAAS, has been implicated in atrial fibrosis and remodelling.39,44,48 Finally, atrial tissue from AF patients has been found to have increased ACE signalling, further implicating the RAAS in the development of atrial fibrosis.
Of clinical significance, ACE-inhibitor treatment has been found to reduce atrial remodelling in AF and has been applied therapeutically to reduce AF in hypertensive and heart failure patients.49–52
Medical Attention
Patients with CKD spend more time in direct contact with healthcare professionals than healthy people of a similar age. This is particularly marked for those receiving haemodialysis or awaiting renal transplantation.
Arrhythmias in this group should be diagnosed promptly and referred appropriately. Dialysis gives an exceptional opportunity to observe the heart rhythm under conditions of haemodynamic stress; effectively, it is a twice-weekly provocation test for a large cohort of vulnerable patients.
Cardiac arrest during haemodialysis occurs at a rate of 1–7.5 per 100,000 haemodialysis sessions, so there is an opportunity to intervene and save lives.53–55 Dialysis services are therefore obliged to maintain vigilance and preparedness.
Causality
With so many mechanisms to choose from, the difficulty lies not in determining whether an association exists between CKD and arrhythmias but in determining the most important mechanisms of connection.
A recent bidirectional Mendelian randomisation study attempted to determine the causality involved in the relationship between CKD and AF. The analysis by Park et al. suggested that genetically predicted AF was significantly associated with CKD and a lower estimated glomerular filtration rate (eGFR) with statistically significant causal estimates. They did not detect an effect of genetically determined eGFR on the incidence of AF. This indicated that AF was possibly a causal risk factor for CKD, but not vice versa.56 It is unlikely that AF is a direct cause of CKD; the relationship is likely to be more complex and involve a multitude of mechanisms. However, this study does suggest that there is a link between arrhythmias and CKD.
Managing Arrhythmia in Renal Disease
Management of arrhythmia begins with the accumulation of diagnostic information. The critical step is to collect electrocardiographic documentation at the right moment.
The symptoms of arrhythmia are protean: palpitations, syncope, pre-syncope and chest discomfort may occur in any form of tachyarrhythmia and in any bradyarrhythmia.57 More often, arrhythmias produce just a decline in exercise tolerance, dyspnoea on exertion and general malaise.
Because most arrhythmias are intermittent at their onset, documentation and therefore diagnosis are a challenge. Provided the physician is alert to the possibility of an arrhythmia, electronic devices are available to suit the clinical situation.
The choice of device depends on the frequency and duration of the symptomatic events. Frequent but brief symptoms can be assessed on a 24-hour recording; infrequent events of long duration can be documented by performing a standard ECG when the symptoms are present. Symptoms that are both brief and infrequent may require the implantation of a loop recorder.57
Therapy for a patient with arrhythmia should initially address any modifiable underlying condition and should mitigate the risks associated with the arrhythmia. In all cases, valve disease and myocardial ischaemia should be evaluated and, in general, corrected. Heart failure, if present, should be managed optimally. For the renal patient, correction of underlying causes should include optimisation of the control of renal indices. Mitigation of risks includes rate-limiting therapy for any atrial arrhythmia that of >100 BPM and long-term anticoagulation for many patients with persistent atrial tachyarrhythmias.
With underlying conditions corrected, management of renal problems optimised and the risks of thromboembolic complications mitigated, many patients will experience a resolution of arrhythmia episodes or a resolution of arrhythmia-related symptoms and will not require additional therapy.
For those who experience recurrent or continuing symptomatic episodes, specific therapy is indicated to restore and maintain sinus rhythm. This may involve catheter-based procedures, implanted devices, arrhythmia surgery or specific antiarrhythmic drugs alone or in combination.
Exceptions in Renal Failure
Patients with renal impairment are vulnerable to complications that make their management diverge in important ways from the general population.
Device Therapy in Chronic Kidney Disease
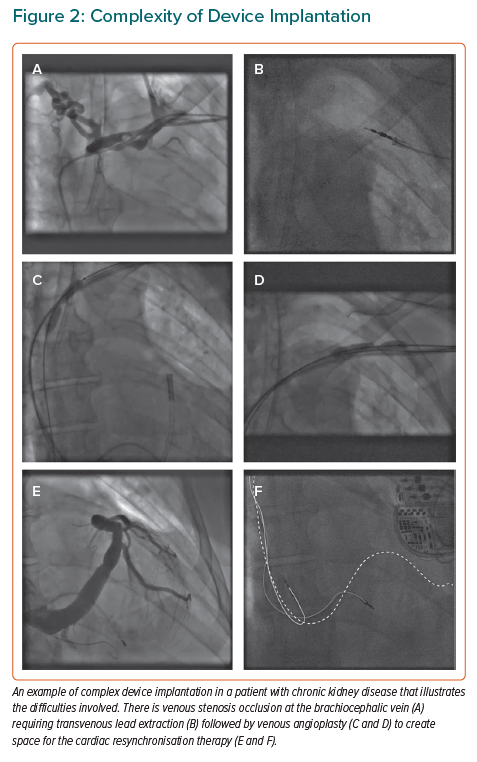
Patients with CKD are difficult subjects for device therapy because of the effects of renal replacement therapy on the venous system (Figure 2). Chronically indwelling catheters commonly cause venous stenosis or occlusion, making those veins difficult or impossible for subsequent lead implantation.
There are important differences between the general population and the CKD population in the risk associated with device therapy, predominantly due to the risk of device infection.
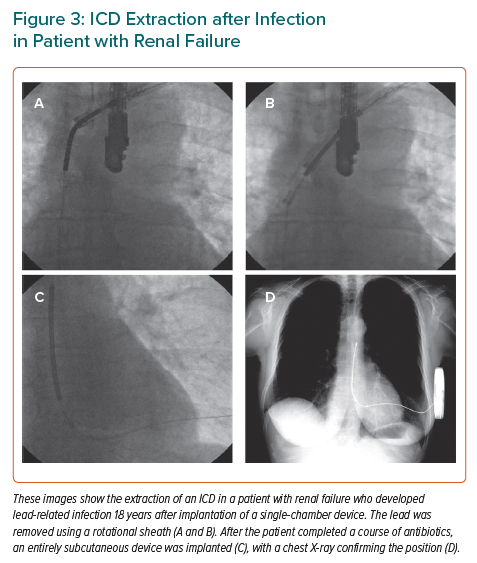
Infection by bacteria introduced during the implantation procedure can manifest as pocket swelling or erosion of the device through the skin at months or years after implantation. More seriously, endovascular infection can result from bacteria introduced at the time of implantation or from bacteria that colonise the leads, often following introduction during dialysis. Once established, either form of infection is near impossible to control without extraction of the device, a substantial undertaking associated with a mortality risk above 0.2% even in the most experienced centres (Figure 3).
The risk of infective complication is significantly higher in patients with renal impairment than in the general population; infection is the second leading cause of death in this cohort.58 A large observational study of 25,675 pre-dialysis patients found that this risk was inversely related to eGFR; the highest risk is associated with the lowest eGFR, with a 3.5-fold higher risk in patients with an eGFR of 30 ml/min/1.73 m2.59
The picture is bleaker for patients on dialysis. The HEMO study, a randomised controlled trial involving 1,846 patients, examined the effects of dialysis dose and flux on patient outcomes found that the there was a 35% annual hospitalisation rate for infection in this group. The risk of infection-related mortality was also found to be high in this subgroup; 23.1% of all deaths in this study were infection related whilst 58% of patients with infection-related first hospitalisation were associated with a severe outcome (death, intensive care stay or prolonged hospital admission).60
Renal impairment has been identified as a potent risk factor for infection in patients with cardiac implantable electronic devices.61 For a patient with CKD, the risk of death from device infection is approximately three times higher, enough to influence the risk-benefit calculation that drives decision-making in device therapy.62 Many rules of thumb used in the general population are therefore not valid in CKD.
ICD therapy is widely used in patients assessed to have a risk of sudden death of >1% per year. Patients with severe impairment of LV systolic function without a reversible cause generally fit this criterion and receive ICD therapy. This is based on the findings of major clinical trials that have demonstrated clear benefit in this cohort of patients.63–65
In CKD, the evidence is much less clear; the major ICD trials routinely excluded patients with CKD and the conclusions may therefore not apply to this subgroup. Early evidence suggests that ICD therapy does not benefit patients with loss of kidney function. A retrospective analysis of 61 patients with CKD recruited in MADIT-II did suggest a survival benefit with ICD therapy in patients with an eGFR of >35 ml/min/m2; however, there was no benefit in patients with an eGFR of <35 ml/min/m2 .
There is evidence that CKD increases the risk of death in patients receiving an ICD. An observational study of 507 consecutive patients with varying stages of CKD receiving a novel ICD implant found a risk of mortality with renal impairment that increased stepwise by eGFR stage; renal dysfunction was independently associated with mortality in patients receiving an ICD.66
This was validated by subsequent meta-analyses, which concluded that CKD in patients with an ICD significantly increased the risk of mortality and suggested that this risk is comparable between earlier stages of renal insufficiency to end-stage renal disease.67,68
A study involving two separate meta-analyses performed by Makki et al. evaluated the effect of CKD on ICD and ICD on CKD patient outcomes. The authors concluded that ICD patients have a higher risk of dying if they have CKD in comparison to those ICD patients who did not have CKD. Conversely, CKD patients have a lower risk of mortality (from SCD) with an ICD, comparatively to those CKD patients who did not have an ICD fitted.69
A subsequent randomised controlled trial of 188 patients on dialysis with a left ventricular ejection fraction of >35% found that prophylactic ICD therapy did not reduce mortality from sudden cardiac death compared to not receiving this therapy.70 Despite this, 13.8% of the ICD group received appropriate ICD therapy for ventricular arrhythmias and there was an overall lower incidence of SCD (10.1%) than in previous reports (22–26%).70 There are notable limitations to this trial: the ICD was implanted in patients with no class I indication so the risk of SCD was lower. The population was also well optimised before enrolment, which may have protected against SCD.
The classification of SCD is difficult, especially in the absence of any cardiac monitoring during the terminal event. It is assumed arrhythmic if the patient’s death was sudden, unwitnessed and the patient was well when last observed. Therefore, it is difficult to accurately assess endpoint in the two subgroups.70
ICD therapy is, therefore, reserved for those at highest risk of arrhythmia-related death, including survivors of a cardiac arrest due to ventricular tachycardia or ventricular fibrillation.
When an implanted device is required, the presence of CKD has an important influence on the choice of methodology and equipment. Mitigation of the risk of infection is the key objective; because the greatest modifiable risk is the seeding of bacteria to device surfaces exposed to the vascular space, measures are taken to minimise the exposed surface area.
In the case of ICD therapy, intravascular components can be eliminated completely, using solely components that lie in the subcutaneous space. These devices lack the capability to treat bradycardia that is universal in transvenous devices but, in many cases, the risk of fatal bradyarrhythmia is less than the risk of fatal infection.
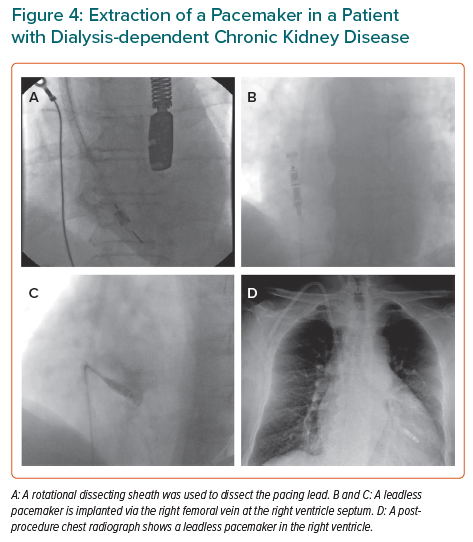
Pacing therapy can be delivered through a leadless system (Figure 4); although this lies in the vascular space, it benefits from having a surface area far less than that of a pacing lead.
Procedural Risks and Benefits of Ablation
Arrhythmias are very managed definitively by catheter-based procedures. Circuits are destroyed by radiofrequency (RF) energy, pulsed field energy, laser or cryotherapy delivered by catheters placed via the femoral vessels or, less often, epicardially.
For most symptomatic and recurrent arrhythmias, catheter ablation is the accepted firstline therapy. Catheter ablation has become one of the most common medical procedures, with most of these performed for AF.
Ablation for AF in patients with CKD carries a greater risk than for other patients, including a risk of acute exacerbation of renal dysfunction, but there is evidence that the procedure can result in improved renal function over the long term.71
A prospective study of 386 patients who underwent AF ablation revealed that eGFR improved with restoration of sinus rhythm within 3 months of the procedure and was maintained up to 1 year after the ablation. Patients with arrhythmia recurrence demonstrated a reduction in the renal function.72
Recurrence following AF ablation is not uncommon in patients with CKD. In a study of 221 patients with a mean follow-up of 32 months following AF ablation therapy, CKD patients had a significantly higher incidence of AF recurrence than non-CKD participants; CKD was identified as independent associated variate with AF recurrence.73
This is consistent with a recent meta-analysis of seven observational studies, which concluded that CKD was significantly associated with higher AF recurrence than in to non-CKD patients (OR 3.71).74
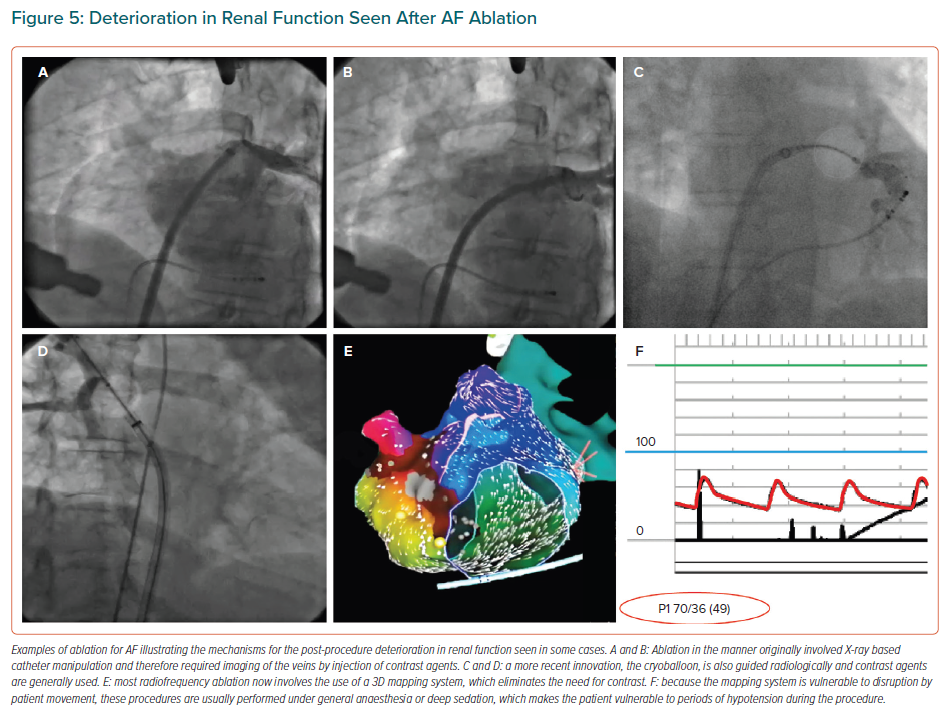
The kidneys are vulnerable to injury from use of radiological contrast media. Early methods of RF ablation for AF necessitated the use of contrast.75 Current RF methods rely on 3D mapping systems, which obviate the need for this medium (Figure 5) but create a need for general anaesthesia or deep sedation combined with analgesia, which can create hypotension severe enough to injure the kidneys.
Cryotherapy is comparable in efficacy to RF methods, but usually involves the use of contrast agents.76 RF ablation without contrast and with meticulous control of arterial pressure is the preferred method.
Pharmacokinetics
Antiarrhythmic drug therapy with agents that modify the function of ion channels can be used selectively in the management of certain arrhythmias, usually as a bridging measure until an underlying condition is corrected or definitive therapy can be offered. This group of drugs has been shown to increase all-cause mortality in a number of studies in different patient populations, so their long-term use as the sole management strategy has diminished.77–79
There are insufficient data to determine specifically the risks of antiarrhythmic drugs in patients with CKD, but the altered and unpredictable pharmacokinetics of the renal failure state would be expected to augment the risk.
Because these medications act on sodium and potassium channels, the exaggerated fluctuations in ion concentration associated with CKD and dialysis could also pose a risk.
Even β-blocking drugs – a group associated with few dangerous adverse effects in the general population – can cause serious adverse effects in the context of CKD, where altered kinetics combined with electrolyte disturbance can trigger BRASH syndrome.
Bleeding
Bleeding complications are much more common in CKD, and are probably an effect of platelet dysfunction combined with the consequences of hypertension and direct vascular effects.
Bleeding complications occurring at the time of device implantation or ablation account for some of the excess risks of these therapies in CKD patients.80 The risk of spontaneous bleeding, most importantly intracranial bleeding, is high enough to move the balance of risk associated with the use of long-term anticoagulants.
In the general population, AF combined with one other risk for thromboembolism creates a risk that is great enough to justify the haemorrhagic risk of long-term anticoagulation for most patients. In patients with CKD, the increased risk of bleeding is sufficient to outweigh the risk of thromboembolism such that anticoagulation is reserved for those at highest risk of thromboembolism.
When anticoagulants are required, choice is restricted in the CKD population. Warfarin and other vitamin-K antagonists have been almost entirely displaced by direct oral anticoagulant (DOAC) drugs in the general population,based partly on evidence of a safety benefit but mostly due to the inconvenience of the blood testing required to make vitamin-K antagonists safe.81–84
These agents rely on renal clearance and so their application in patients with CKD has limitations. In general, patients with a creatinine clearance (CrCl) of <50 ml/min have been recommended to lower the dose of their DOAC, while those with a CrCl clearance of <15 ml/min are advised against their use.85 Despite this dose reduction in moderate renal impairment (CrCl 30–50 ml/min), DOAC agents have been shown to be safe and efficacious with a comparable bleeding risk and stroke prevention to warfarin.86
There is evidence suggesting that they may be safe in severe renal impairment also, with some reassuring experience in patients receiving renal dialysis.87–88 A Cochrane review of 12,545 patients assessed the efficacy and safety of DOACs versus warfarin in patients with AF and CKD. Of these, 390 had severe renal impairment (CrCl 15–30 ml/min).89 In keeping with previous evidence, the study concluded that DOACs were as safe and efficacious as warfarin.
Although the study applies mostly to patients with moderate renal failure, it also indicated DOAC use in the severe category was plausible and possibly safe; further work is required. On the basis of current evidence, DOAC therapy is not available to most patients with CKD so the inconvenience of warfarin and similar agents is an added reason to avoid anticoagulation.
Left atrial appendage occlusion, by a catheter-based procedure or removal or occlusion of the appendage by minimally invasive surgery, has the potential to resolve the dilemma of stroke risk and bleeding risk in CKD patients. These interventions have been shown to have a similar efficacy to anticoagulation in preventing stroke in the general AF population, and the catheter-based approach compares favourably to either anticoagulation or no therapy in dialysis patients.90
Economic Impact of Arrhythmias in CKD
The economic impact of AF in CKD is difficult to quantify as it is a cumulative effect. Catheter ablation is expensive, although the costs are improving.
A study based on a registry of 12,027 patients found that catheter ablation was relatively expensive, with first procedure success associated with a significantly lower cost than repeat ablations.91 As the likelihood of arrhythmia recurrence is higher in CKD patients, this is significant.
In comparison to pharmacotherapy, however, catheter ablation is an economically viable option. A cost-effectiveness systematic analysis comparing catheter ablation with pharmacotherapy for AF revealed that ablation therapy in the medium-to-long term was more cost-effective than medical therapy.92 This is probably influenced by the number of repeated hospital admissions associated with arrhythmias managed with pharmacological agents as well as the overall cost of these agents over the lifetime of a patient.
In patients with CKD, medical therapy with anti-arrhythmic agents is also difficult because of the side effects associated with them; the majority have proarrhythmic effects and the metabolic imbalances in CKD are likely to compound this further.
Cardiac rhythm management with device therapy also has upfront costs. In the heart failure patient, device therapy requires careful consideration of benefit and cost. In the CKD population, this is amplified as this cohort has an overall lower life expectancy than the general population.93
The evidence of benefit is also not as clear. There are additional risks to consider, including device infection and consequent treatments including transvenous lead extractions (Figure 6), which are costly. Leadless systems are increasingly prevalent and, although they carry a smaller risk of transvenous infections, they are far more expensive.94
Conclusion
Patients with CKD are vulnerable to arrhythmia for many reasons that are well understood and probably through other less familiar mechanisms. Management of arrhythmia is made more difficult by the presence of severe renal dysfunction, but therapeutic options are available and continue to evolve. Optimal management of arrhythmia not only improves the quality of life of many patients but can, in some cases, extend life and slow the progression of CKD.