Hypertension is the most prevalent cardiovascular disease affecting 20–50 % of the adult population.1 Elevated blood pressure has been identified as a risk factor for coronary heart disease (CHD), heart failure, stroke, peripheral arterial disease, renal failure and atrial fibrillation both in men and women in a large number of epidemiological studies.2–4 So far, the largest meta-analysis of randomised trials of blood pressure reduction has shown that lowering systolic blood pressure by 10 mmHg and diastolic blood pressure by 5 mmHg using any of the main classes of blood pressure-lowering drugs reduces CHD events and heart failure by about a quarter, and stroke by about a third.5 The benefit of antihypertensive medication is due mostly to blood pressure lowering per se.
Hypertension often clusters with other cardiovascular (CV) risk factors associated with increased risk of CV events. Hypertension and dyslipidaemia are two major CV risk factors highly prevalent either alone or in combination.6 Due to interaction of CV risk factors, the probability of a CV event is frequently greater in patients with only moderate blood pressure and cholesterol abnormalities in the presence of additional risk factors.
A major aim of treating hypertension is a maximal decrease of long-term total CV risk, which could only be achieved by treatment of all reversible risk factors and associated conditions in addition to treatment of raised blood pressure per se.
The aim of this review is to analyse whether there is further benefit from antiplatelet and lipid-lowering drugs in hypertension.
Antiplatelet Drugs in Hypertension
As hypertension is associated with increased intravascular pressure, most of the expected complications should be of haemorrhagic origin; however, most of the hypertension-related complications in developed countries are nowadays thrombotic ones, with CHD and ischaemic stroke being the most prevalent events. In addition, some hypertension complications such as heart failure or atrial fibrillation are themselves associated with increased risk of stroke and thromboembolism. Therefore, antithrombotic therapy should possibly reduce thrombotic complications in hypertensive patients.
Hypertension Optimal Treatment
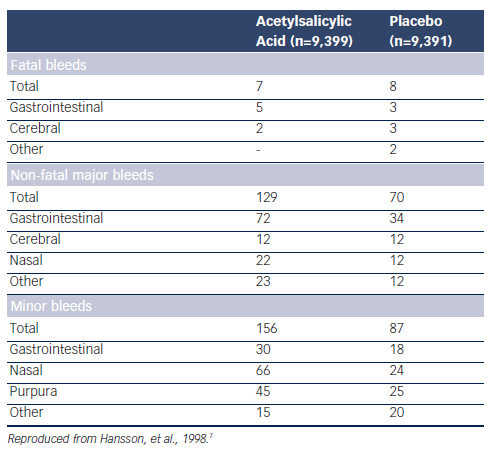
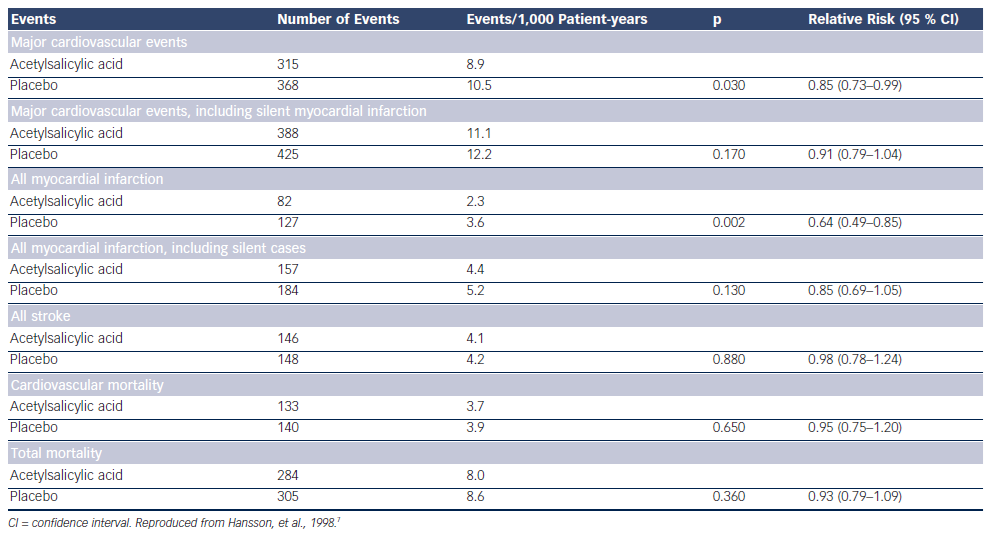
So far the only study assessing the potential benefit of a low dose of acetylsalicylic acid (ASA) in hypertension is the Hypertension Optimal Treatment (HOT) randomised trial.7 A total of 18,790 patients aged 50–80 years were randomly assigned to three target diastolic blood pressures: ≤90 mmHg, ≤85 mmHg and ≤80 mmHg. Within each group, the patients were randomised to 75 mg/day ASA or placebo. Low-dose ASA reduced major CV events and all myocardial infarctions (see Table 1). Fatal bleeds including cerebral ones did not differ in the two groups but non-fatal major and minor bleeds were significantly more frequent among patients receiving ASA than in those taking placebo (see Table 2).
Before the publication of the HOT study, hypertension was often considered a contraindication to ASA because of the potentially increased risk of bleeding (cerebral in particular).7
A later publication from the HOT study showed gender differences in the preventive effect of ASA, which significantly reduced myocardial infarction only in men by 42 %; but the reduction in myocardial infarction was only 19 %, and thus not significant in women. This was due to less statistical power when subdivided into men and women.8
Subgroup-treatment interaction analyses indicated that only patients with serum creatinine >1.3 mg/dl had a significantly greater reduction of CV events and myocardial infarction, while the risk of bleeding was not significantly different between the subgroups.9 A favourable balance between benefit and harm of ASA was documented in subgroups of patients at higher global baseline risk and baseline systolic blood pressure (≥180 mmHg).
More recently, the benefit of ASA was significantly greater in a subgroup with low estimated glomerular filtration rate (eGFR) (<45 ml/min/1.73 m2), for which an increased risk of major bleeding appears to be outweighed by substantial benefit.10
Cochrane Collaboration Review of Antiplatelet Agents For Hypertension
Lip et al. found four trials including a total of 44,012 patients to be subject of the meta-analysis.11 ASA did not reduce stroke or all CV events compared with placebo in primary prevention patients with elevated blood pressure and no prior CV disease. On the other hand, myocardial infarction was reduced with ASA in primary prevention; however, the benefit was negated by harm of similar magnitude due to an increase in major haemorrhage.
The benefit of antiplatelet therapy for secondary prevention in patients with hypertension is many times greater than the harm. No benefit for warfarin therapy alone or in combination with ASA was found in patients with elevated blood pressure. Diclopidine, clopidogrel and newer antiplatelet agents (prasugrel, ticagrelor) have not been sufficiently evaluated in patients with hypertension.
There is a need for further trials evaluating antithrombotic therapy, including newer agents in hypertension.
2013 European Society of Hypertension/European Society of Cardiology Guidelines
The 2013 European Society of Hypertension (ESH)/European Society of Cardiology guidelines12 concluded that antiplatelet therapy, particularly low-dose ASA, should be prescribed to controlled hypertensive patients with previous CV events and should be considered in hypertensive patients with reduced renal function or a high CV risk. ASA is not recommended in low-to-moderate risk hypertensive patients in whom absolute benefit and harm are equivalent.
Acetylsalicylic Acid in Preventing Pre-eclampsia
Pre-eclampsia (defined as de novo appearance of hypertension in pregnancy accompanied by proteinuria >0.3 g/24 hours) is associated with increased risk of maternal, foetal and neonatal morbidity and mortality. A reliable prediction of development of this condition has so far failed. A meta-analysis by Duley et al.13 showed only a mild risk reduction of developing pre-eclapmsia with low-dose ASA. Therefore, low-dose of ASA was only recommended in pregnant women at high risk of developing pre-eclampsia defined as a history of pre-eclampsia presenting before 28 weeks of gestation.14
In 2010, Bujold et al. pooled data from over 11,000 women enrolled in randomised controlled trials evaluating low-dose ASA in the treatment of pregnant women at moderate or high risk for preeclampsia.15 They concluded that women who initiated treatment at less than 16 weeks of gestation had a relative risk (RR) of 0.47 (confidence interval [CI] 0.34–0.65) for developing pre-eclampsia, and a 0.09 RR (CI 0.02–0.37) for developing severe pre-eclampsia compared with controls. Women at high risk of pre-eclampsia (hypertension in a previous pregnancy, chronic kidney disease, autoimmune disease such as systemic lupus erythematosus or antiphospholipid syndrome, type 1 or 2 diabetes, chronic hypertension) or with more than one moderate risk factors for preeclampsia (first pregnancy, age ≥40 years, pregnancy interval of >10 years, body mass index (BMI) ≥35 kg/m2 at first visit, family history of pre-eclampsia and multiple pregnancy) are advised to take 75 mg of ASA daily from 12 weeks until delivery.16
Lipid-lowering Drugs in Hypertension
Of all the lipid-lowering drugs, only statins have been properly tested in large clinical trials in hypertensive patients showing their ability to reduce CV morbidity and mortality.17
Effects of Statins on Blood Pressure and Renal Function – Pathophysiological Mechanism
By blocking the synthesis of 3-hydroxy-3-methylglutaryl coenzyme A reductase, statins induce consistent and predictable reductions in circulating LDL-cholesterol and triglycerides, and have a small effect on high-density lipoprotein (HDL)-cholesterol. In addition, these agents exhibit ancillary actions attributed to reductions in isoprenoid cholesterol intermediates and reductions in dolichols, geranylgeranoic acid and farsenylfarsenoic acid. These actions may provide a pleiotropic mechanism by which statins exert actions on blood pressure as well as target organ damage associated with hypertension. Statins improve endothelial function by increasing the bioavailability of nitric oxide, promoting reendothelialisation, reducing oxidative stress and inhibiting inflammatory responses.18 Increased angiotensin II sensitivity predisposes to hypertension and plaque instability. The increased sensitivity to angiotensin II in healthy young subjects with isolated hypercholesterolaemia can be partly restored by therapy to reduce the levels of LDL-cholesterol using statins. There is evidence that statins downregulate angiotensin II type 1 (AT1)-receptor expression.19
Clinical trials have demonstrated that aggressive treatment with statins improves serum creatinine, glomerular filtration rate and urate levels.20,21 This effect is probably another consequence of improved blood flow following treatment with statins. The beneficial effect of statins in preventing renal dysfunction has also been documented and seems to be independent of their lipid-lowering effect.22 Statins significantly reduce albuminuria or proteinuria and are associated with a small reduction in the rate of kidney function loss, particularly in populations with CV disease.23
Effects of Statins on Blood Pressure in Clinical Studies
Most of the studies report a small reduction in blood pressure; however, the blood pressure-lowering effect of statins is not consistent.
The effect on blood pressure (BP) varied from neutral to most favourable (Δ systolic BP 8–13 mmHg; Δ diastolic BP 5.0–7.8 mmHg) in a review by Milionis et al., including studies within a broad spectrum of patients (normotensives, hypertensives, individuals with normal lipids and dyslipidaemia, diabetic patients) published up to 2005.24
A meta-analysis of all studies published up to 2005 and reporting BP data during treatment with statins included 20 randomised controlled trials (828 patients) lasting from one to 12 months.25 Systolic BP was significantly lower in patients on statins than in those on placebo or a comparative lipid-lowering drug (mean difference: -1.9 mmHg; 95 % CI -3.8 to -0.1). The effect was greater when the analysis was restricted to studies with a baseline systolic BP >130 mmHg (Δ systolic BP -4.0 mmHg; 95 % CI -5.8 to 2.2). There was a trend toward lower diastolic BP in patients receiving statin therapy compared with controls: -0.9 mmHg (95 % CI -2.0 to 0.2) overall and -1.2 mmHg (95 % CI -2.6 to 0.1) in studies with a baseline diastolic BP >80 mmHg.
The University of California San Diego (UCSD) Statin Study, a randomised, double-blind, placebo-controlled trial with 973 patients allocated equally to simvastatin (20 mg), pravastatin (40 mg) or placebo for six months, showed a modest but significant BP reduction (2.4–2.8 mmHg for both systolic blood pressure [SBP] and diastolic blood pressure [DBP]) with both statins.26 As this effect was seen in patients not receiving antihypertensive treatment (most patients were normotensive), these results are compatible with the above possibility that statins exert a small BP-lowering effect that can be detected only when they are given alone.
By contrast, in the Plaque Hypertension Lipid-Lowering Italian Study (PHYLLIS), a randomised, placebo-controlled, double-blind study including 508 patients with mild hypertension and hypercholesterolaemia, administration of a statin (pravastatin 40 mg once daily) in hypertensive patients with BP effectively reduced by concomitant antihypertensive treatment did not have an additional BP-lowering effect.27 The strengths of this study were a 2.6-year follow-up and ambulatory BP monitoring in addition to clinic BP measurement.
A recent meta-analysis of 40 studies and 51 comparison groups (22,511 controls and 22,602 patients) reported a decrease in mean SBP in the statin group by 2.62 mmHg (95 % CI -3.41 to -1.84; p<0.001) and DBP by 0.94 mmHg (95 % CI -1.31 to -0.57; p<0.001). In studies including hypertensive patients, the decrease in BP with statins was slightly greater (SBP -3.07 mmHg; 95 % CI -4 to 2.15 and DBP 1.04 mmHg; 95 % CI -1.47 to -0.61).28
Studies in Blood Pressure – Large Clinical Outcome Trials
Statins and Blood Pressure – Implications of Large Clinical Outcome Trials
Treatment of hypertension is associated with a reduction in stroke and, to a lesser extent, coronary events. It is also well-known that elevated serum total cholesterol significantly increases CHD risk. Therefore, it is logical that co-existing vascular risk factors including abnormal lipid profiles should be an integral part of hypertension management.
The benefit of lowering both BP and cholesterol was evaluated in two large-scale trials: Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT)29 and Anglo-Scandinavian Cardiac Outcomes Trial - Lipid-Lowering Arm (ASCOT-LLA).30
Part of ALLHAT was designed to determine whether pravastatin compared with usual care would reduce all-cause mortality in 10,355 patients with hypertension and moderate hypercholesterolaemia, plus at least one additional CHD risk factor.29 At four years, total cholesterol was reduced by 17.2 % with pravastatin versus 7.6 % with usual care. All-cause mortality was similar in the two groups and CHD event rates were not different between the two groups; six-year CHD event rates were 9.3 % (pravastatin) and 10.4 % (usual care). These results could be attributed to the small difference in total cholesterol (9.6 %) and LDL-cholesterol (16.7 %) between pravastatin and usual care compared with other statin trials. Adherence to the treatment assigned declined over time. For those assigned to pravastatin, adherence dropped from 87.2 % at year two to 80 % at year four, and 77 % at year six, although the number of participants was small. On the other hand, in the usual care group, crossovers to statin treatment increased from 8 % at year two to 17 % by year four. This increase continued at year six, but the number of participants was small.
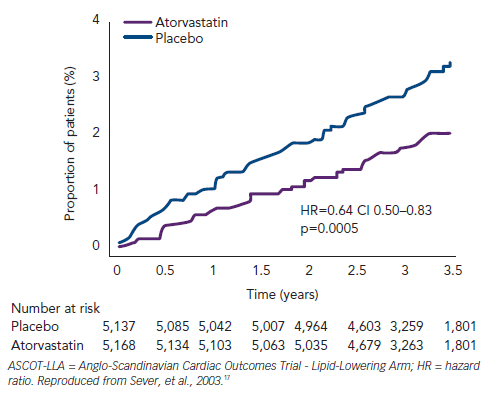
In the Anglo-Scandinavian Cardiac Outcomes Trial - Blood Pressure-Lowering Arm (ASCOT-BPLA) trial,30 19,342 men and women with hypertension and at least three other CV risk factors were randomised to amlodipine (5–10 mg/d) ± perindopril (4–8 mg/d) or to atenolol (50–100 mg/d) ± bendroflumethiazide (1.25–2.5 mg/d). A total of 10,305 of these patients with normal or slightly elevated total cholesterol were randomised to atorvastatin 10 mg/d or placebo.17 The atorvastatin arm was stopped prematurely at 3.3 years due to a significant reduction in the primary endpoint (-36 %; p=0.0005) (see Figure 1). The benefit of atorvastatin treatment was apparent within the first year of treatment. Fatal/ non-fatal stroke and total CV/coronary events were also reduced with atorvastatin. At one year, atorvastatin reduced total cholesterol by 24 % and LDL-cholesterol by 35 %. However, in the period between six weeks and 18 months, a significant 1.1/0.7 mmHg difference in BP was seen in favour of atorvastatin regardless of titration of doses and numbers of drugs. Overall, amlodipine-perindopril therapy was superior to atenolol-bendroflumethiazide therapy,30 and a further analysis of early monotherapy data comparing amlodipine with atenolol suggested a positive interaction between atorvastatin and amlodipine.31 Compared with placebo, allocation to atorvastatin reduced the incidence of the primary endpoint significantly by 53 % (hazard ratio [HR] 0.47; 95 % CI 0.32–0.69; p<0.0001) among those allocated the amlodipine-based regimen, whereas it reduced the incidence of this outcome by only 16 % (HR 0.84; 95 % CI 0.60–1.17; p=0.30). The difference between these risk reductions with atorvastatin was of borderline significance (heterogeneity; p=0.025) (see Figure 2).
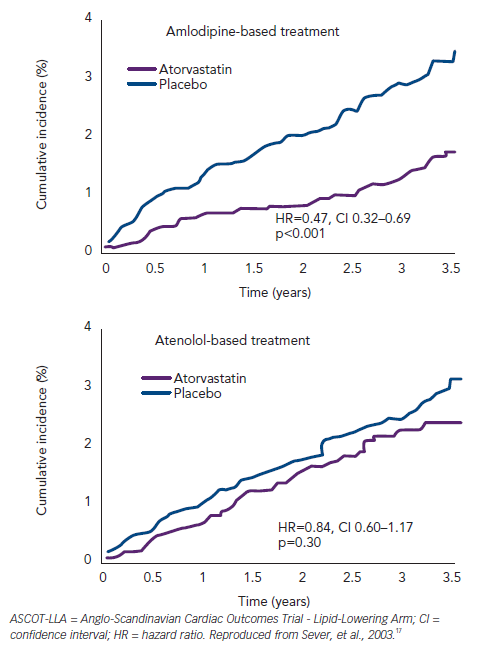
Despite extensive crossovers from and to statin usage, the RR reduction in primary events among those originally assigned to atorvastatin remained at 36 % (HR 0.64; 95 % CI 0.53–0.78; p<0.0001) (carryover effect) 2.2 years after the end of the ASCOT-BPLA.32
In the UK ASCOT population, all-cause mortality remained significantly lower in those originally assigned atorvastatin (HR 0.86, 95 % CI 0.76–0.98; p<0.02) 11 years after initial randomisation and approximately eight years after closure of the lipid-lowering arm (LLA), which may be due to legacy effect.33
A meta-analysis of large clinical trials, including only those with more than 1,000 patients followed for more than two years, was published by Messerli et al.34 Besides ASCOT-LLA and the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT), 12 trials enrolling 69,284 patients met the inclusion criteria. Overall, in these 12 trials, statin therapy decreased cardiac death by 24 % (RR 0.76; 95 % CI 0.71–0.82). There was no evidence of a difference in RR estimates for hypertensive and normotensive patients. In conclusion, statin therapy effectively decreased CV morbidity and mortality to the same extent in hypertensive and normotensive patients.
2013 European Society of Hypertension/European Society of Cardiology Guidelines
The 2013 ESH/ESC guidelines12 recommend using statin therapy in hypertensive patients at moderate-to-high CV risk to achieve the target LDL cholesterol value <3 mmol/l (115 mg/dl). For individuals with manifest CV disease or at very high CV risk35 a more aggressive LDL target of <1.8 mmol/l (70 mg/dl) is recommended.