Up to 50% of patients who undergo elective coronary angiography for stable chest pain symptoms that are mainly related to exercise and typical enough to suggest the presence of obstructive coronary artery disease (CAD) are found to have normal or near-normal coronary arteries.1 The mechanisms responsible for angina chest pain in these patients are heterogeneous; accordingly, their identification is crucial for the tailored management of individual patients.
A large number of studies have shown that most of these patients display abnormalities in the regulation of coronary blood flow (CBF) and coronary vascular resistance (CVR), suggesting that abnormalities of small coronary artery vessels are the cause of symptoms, a condition which is defined as primary stable microvascular angina (MVA), in the absence of other heart disease.2,3
This article focuses on how a diagnosis of MVA could be achieved or excluded in patients presenting with angina chest pain but with an absence of obstructive coronary lesions, and examines issues related to the diagnostic process.
The Diagnosis of Microvascular Angina
Since small coronary arteries cannot be assessed at angiography, in clinical practice the diagnosis of MVA is usually hypothesised after the exclusion of other possible causes of symptoms, both cardiac and non-cardiac. Cardiac causes include both ischaemic (e.g. epicardial spasm; see later) and non-ischaemic diseases (e.g. inflammatory diseases, abnormal stimulation of cardiac nociceptors),4 whereas non-cardiac causes include gastro-oesophageal disorders, in particular gastro-oesophageal reflux,5 as well as musculoskeletal and psychosomatic causes.
However, a definitive diagnosis of MVA requires the documentation of functional abnormalities of the coronary microcirculation.
Methods to Assess Coronary Microvascular Function
Several methods have been proposed to assess coronary microvascular function.6,7 Independent of the method applied, the assessment of the functional state of coronary microcirculation is based on the measurement of CBF and/or CVR at rest and following the administration of vasoactive stimuli, with the effect expressed as the ratio of peak-to-basal values or the percent of variation.
Invasive methods are considered the gold standard to measure the response to vasoactive stimuli of the coronary microcirculation. CBF has classically been derived from CBF velocity measured by intracoronary Doppler wires.8 More recently, an intracoronary thermodilution-derived method has been introduced to measure CBF using a wire that also allows the simultaneous measurement of intracoronary pressure and the calculation of an index of coronary microvascular resistance. In some studies, this method has been found to achieve more reproducible results.9 However, recent data have shown a better correlation of Doppler-derived measurements of coronary flow reserve (CFR) compared with thermodilution-derived measurements, with CFR assessed by non-invasive methods, such as PET and cardiac MRI (CMR; see later) suggesting that they show more reliable results on coronary microvascular function.10,11 Invasive methods, however, present with some limitations, including the prolongation of diagnostic angiography and an increase in cost and risk.
In the absence of obstructive CAD, coronary microvascular dilator function can reliably be investigated by several non-invasive methods that allow measurement of CBF.
Cardiac PET is perhaps the most reliable method at present, and has been applied in several studies in patients with suspected MVA.12,13 PET allows quantitative measurements of myocardial blood flow (MBF; both global and regional) using myocardial distribution and radioactivity of tracers such as 15O-water, 13N-ammonia, and 82rubidium.6,7,10
CMR also allows for reliable assessment of coronary microvascular function in patients with angina and normal/near-normal coronary arteries, using the paramagnetic contrast medium gadolinium to measure MBF.11,14,15 Compared with PET, CMR has the advantage of being radiation free and having a higher spatial resolution; however, post-acquisition processing is more complicated, artefacts are more frequent, and gadolinium should be avoided in patients with renal failure.6,7 The use of PET and CMR to routinely assess coronary microvascular function in clinical practice is mainly challenged by limited availability and high costs.
Myocardial contrast echocardiography is an attractive method used to assess coronary microvascular function, as it is based on using gas-filled microbubbles as echo-contrast, a largely available and inexpensive echocardiographic technique used to measure MBF. Although found to be reliable in some studies, its diffusion has been restrained by some limitations, including operator dependence, difficulty in obtaining reliable images in some conditions (e.g. obesity, pulmonary disease), and some unresolved technical issues.6,7,16,17
In some studies on patients with suspected MVA, coronary microvascular function has been assessed using transthoracic Doppler echocardiography.17,18 In this method, blood flow in the mid-part of the left anterior descending coronary artery is imaged by colour-Doppler using a high-frequency ultrasound probe (7–10 MHz) and CBF velocity is measured by the pulsed Doppler technique. Transthoracic Doppler echocardiography is a potentially largely applicable method as it is easily available and inexpensive.19 Limitations include operator dependence and the inability to obtain good echocardiographic windows in some patients.6
Assessment of Coronary Microvascular Dilatation
Since chest pain in patients with a suspected stable MVA is mainly induced by physical efforts, it seems reasonable to investigate whether an impairment of dilatation of resistance coronary arteries, limiting the increase of CBF required to match the enhanced myocardial oxygen requirements, is present.
CBF is regulated at the microvascular level by multiple mechanisms, including metabolic, neural, humoral, and mechanical (shear stress) factors.20 When required, coronary microvascular dilatation can be achieved by using various substances to induce a direct relaxant effect on the smooth muscle cells (SMCs) of resistance arteries. Other stimuli, however, result in microvascular dilatation indirectly by inducing the release of dilator substances from the endothelium (mainly nitric oxide [NO]) that eventually act on SMCs. Thus, an impairment of maximal dilatation of the coronary microcirculation may result from either a reduced response of SMCs to dilator stimuli, impaired production and/or release of dilator substances by the endothelium (endothelium-dependent dilatation), or both.5
In typical patients with a suspicion of MVA, an assessment of CBF response to exercise would be ideal to assess coronary microvascular dilatation. However, the measurement of CBF during maximal exercise presents practical issues, both with invasive and non-invasive methods. Atrial pacing might be an alternative stimulus to assess CBF response to increased myocardial oxygen consumption, but it also presents with practical issues. Thus, coronary microvascular dilatation is usually assessed by measuring CBF in response to dilator pharmacologic substances.
Endothelium-independent Coronary Microvascular Dilatation
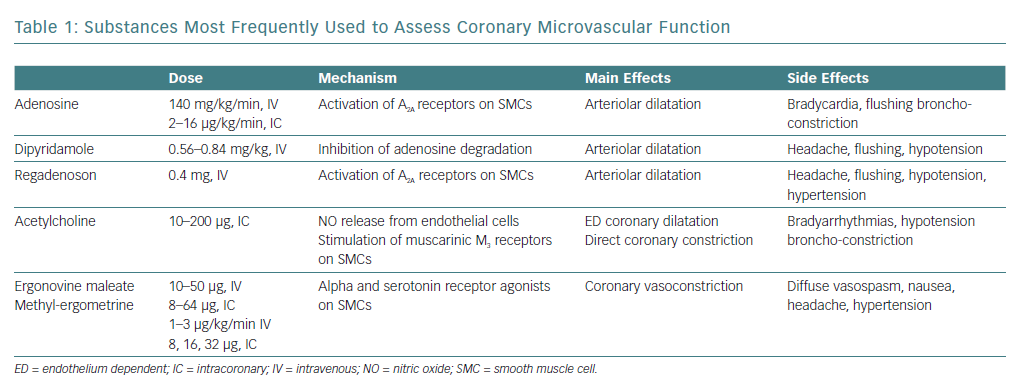
Although various substances (e.g. papaverine, dobutamine, organic nitrates) have been used to investigate the intrinsic dilator capacity of the coronary microcirculation, the arteriolar dilator adenosine and its agonists, dipyridamole and (more recently) regadenoson, are used most frequently (Table 1).21–23
A CFR (ratio between CBF at peak drug administration and at baseline) <2.0 definitively identifies coronary microvascular dysfunction (CMD), whereas an increase in CBF between >2.0 and <2.5 is of borderline significance.
It should be underscored that impaired dilatation of small coronary arteries might originate from functional abnormalities, structural alterations (e.g. SMC hypertrophy, medial fibrosis, intimal thickening), or both.24,25
Endothelium-dependent Coronary Microvascular Dilatation
Endothelium-dependent coronary microvascular dilatation is usually assessed invasively using an acetylcholine test (Table 1). In normal subjects, intracoronary acetylcholine at low-medium doses (usually 10–50 µg) causes microvascular dilatation through the release of NO by endothelial cells,26 with a mild dilator effect also seen on epicardial vessels.
In patients with endothelial dysfunction, the release of NO induced by acetylcholine is impaired, thus resulting in lower degrees of dilatation of small coronary arteries, as indicated by a lower increase in CBF and/or reduction in CVR. Moreover, in case of severe endothelial dysfunction, acetylcholine may actually cause microvascular constriction, as documented by a reduction in CBF and/or increase in CVR.27,28 Through stimulation of muscarinic receptors, acetylcholine also exerts a direct vasoconstrictor effect on SMCs that in normal subjects is masked by the prevailing endothelium-mediated dilatation, but which may become apparent in case of severe endothelial dysfunction.29
There is no clear definition of impaired endothelium-dependent coronary microvascular dilatation with the acetylcholine test, but it has been suggested that failure to increase CBF by >50% should be considered indicative of an impaired dilator response of the coronary microcirculation.30,31
Coronary microvascular endothelial function has also been assessed using other stimuli, in particular the cold pressor test (CPT), which comprises placing a hand in ice for 90 to 120 seconds. The sensation of cold and the accompanying hand pain cause a mild sympathetic activation that slightly increases heart rate and blood pressure; the resulting mild increase in myocardial oxygen consumption determines arteriolar dilation and flow-mediated (endothelium-dependent) dilatation of pre-arteriolar vessels. Furthermore, NO release may also result from the stimulation of endothelial alpha-adrenergic receptors.32 Normal values of CBF response to the CPT have also not been well defined, but we have found that a CBF velocity increase on transthoracic Doppler echocardiography >1.56 allowed a clear discrimination between healthy subjects and MVA patients.17
Microvascular Versus Epicardial Spasm
Some studies have suggested that, at least in some patients, stable MVA might be related to increased coronary microvascular constriction/spasm rather than impaired dilatation. A significant reduction in CBF or increase in CVR has been shown in response to potentially constrictive stimuli, including acetylcholine, hyperventilation, and mental stress, in the absence of any flow-limiting epicardial constriction.27,28,33
Importantly, some studies have recently shown that a sizeable proportion of patients with a suspicion of stable MVA develop typical angina and ischaemic electrocardiographic (ECG) changes in the absence of any significant epicardial spasm in response to higher doses of acetylcholine (up to 200 µg), indicating the induction of coronary microvascular spasm.34–36 Accordingly, it has been suggested that the identification of microvascular spasm as a mechanism of angina symptoms should be achieved by this method rather than by CBF/CVR measurements.37 Importantly, the same doses of acetylcholine have been shown to trigger epicardial spasm in more than 60% of patients, suggesting that this mechanism – rather than microvascular spasm – could be a cause of angina symptoms in a subgroup of patients.34–36 ,8
Of note, the fact that both coronary microvascular and epicardial spasm have been described in patients with stable angina but no obstructive CAD makes it necessary to perform vasoconstrictive tests during invasive coronary angiography to establish the site of the spasm. The tendency towards coronary spasm might also be assessed non-invasively (e.g. by ergonovine test; Table 1), although in this case a positive test will leave doubts about the site of the spasm.39,40
Combined Functional Coronary Alterations
Current data suggest that most patients with angina and no obstructive CAD present with a variable combination of abnormal dilator and constrictive provocative tests.
In a 2011 study, we found an impairment of coronary microvascular dilatation to both adenosine and CPT in 44% of 71 patients with a suspicion of MVA, whereas 21% and 10% of patients presented with an impairment of coronary microvascular dilatation in response to either adenosine or CPT, respectively.17 In a study by Sara et al., an abnormal coronary microvascular response to both adenosine and acetylcholine was found in 36.1% of 1,439 patients, whereas a discordant response was found in 45.2% of patients.41 Finally, in the recently published CORonary MICrovascular Angina (CorMicA) trial, 20.5% of patients had evidence of both impaired response to adenosine and a positive acetylcholine test, while epicardial spasm was induced in 16.5% and CMD (either impaired dilatation or microvascular spasm) in 51.6% of 151 patients.36
Thus, a complete characterisation of CMD and functional abnormalities of coronary circulation in individual patients requires assessment of all the types of tests described above, which might have implications on the choice of specific or combined forms of treatment.
Limitations in the Interpretation of Provocative Tests
We should be aware that, in contrast to current beliefs, there are significant pitfalls in the interpretation of provocative coronary tests and, therefore, the accurate characterisation of coronary alterations.
Thus, the stimuli used to assess endothelium-dependent dilatation are not specific to these tests. Acetylcholine, as discussed above, has also vasoconstrictor effects, and it is not possible to exclude that this effect contributes to the abnormal coronary microvascular response detected by its administration in MVA patients, possibly resulting from an increased reactivity of SMCs. Similar considerations apply to other endothelium-dependent dilator stimuli, such as the CPT, which might trigger spasm in hyperreactive coronary segments through adrenergic activation.42
It should be also observed that, in the presence of a global impairment of SMC relaxation in response to vasodilator substances, normal endothelial release of NO also results in a lower dilator response, thus leading to an erroneous diagnosis of impaired endothelium-dependent dilatation.
On the other hand, the increase in CBF determined by direct arteriolar vasodilators may also depend on endothelium-dependent, flow-mediated pre-arteriolar dilatation, and adenosine, in particular, may in part also act through endothelial NO release.43 Thus, in most cases it is not possible to attribute CMD with certainty to only one of the specific mechanisms that can be responsible for its occurrence.
Finally, it should be observed that, while the induction of epicardial or microvascular spasm in patients with stable angina and no obstructive CAD indicates the presence of an abnormal coronary reactivity, the actual role that these abnormal responses have in determining the angina symptoms in individual patients remains to be demonstrated, particularly when considering epicardial spasm induced by high doses of acetylcholine. Of note, being a vagal neuromediator, acetylcholine is not an ideal stimulus to prove that clinical exercise-induced angina is related to spasm. Some studies have assessed the effect of exercise on coronary vascular reactivity in patients with suspected MVA, consistently showing some degree of vasoconstriction in epicardial vessels in subgroups of patients; none, however, reported exercise-induced occlusive/subocclusive epicardial spasm.44,45 Thus, to avoid false-positive diagnoses of epicardial spasm, only low-to-medium doses of acetylcholine should be used to assess the functional abnormalities of coronary circulation in stable angina patients with no obstructive CAD.
Microvascular Angina Versus Stable Angina
Diagnosis of primary stable MVA presupposes a lack of obstructive CAD in patients with a stable pattern of chest pain. Importantly, while until a few years ago this could be achieved only by invasive coronary angiography, the documentation of normal (or near-normal) coronary arteries can now reliably be obtained by non-invasive angio-CT scan.
Accordingly, in clinical practice, a non-invasive CT coronary angiography can be recommended to define the coronary picture in symptomatic patients with a high probability of MVA. This would avoid the small risk as well as higher costs related to a more invasive procedure. As suggested above, the documentation of CMD to support the diagnosis of MVA may in these cases be achieved by non-invasive methods (see above).
Although a differentiation between classical stable angina and stable MVA is often difficult, a few documented clinical features and results of non-invasive diagnostic tests may help orient the diagnosis towards one of the two forms of angina.
Clinical Features
While chest pain in MVA patients is often indistinguishable from that of CAD patients, two main features, when present, strongly suggest MVA: the persistence of dull chest discomfort several minutes after stopping exercise, in spite of the resolution of typical chest pain; and slow resolution of chest pain after taking a short-acting nitrate.46
Non-invasive Diagnostic Tests
ECG Exercise Stress Test
While the characteristics of ST-segment changes induced during an exercise stress test do not usually allow for a reliable distinction between MVA and CAD patients, the lack of improvement or, even more, a worsening of ischaemic changes during the exercise stress test performed after a preventive administration of short-acting nitrates, would strongly suggest MVA.47,48 For example, in a recent study we found that the time and/or rate pressure product at the ischaemic threshold (1 mm ST-segment depression) was lower than ≥60 s >1,500 BPM*mmHg on the post-nitrate exercise stress test, compared with a baseline test, in 24% of MVA patients, but this was not the case in any CAD patients.49
Echocardiographic Stress Tests
Significant clues to the differential diagnosis of MVA and stable CAD may come from echocardiographic exercise or a pharmacological stress test. The induction of angina and ischaemic ST-segment depression in the absence of reversible regional left ventricle wall motion abnormalities strongly supports the diagnosis of MVA.50–52 The reason for the variable behaviour of left ventricle contraction in the two conditions can be related to the fact that myocardial ischaemia caused by obstructive stenoses of epicardial vessels usually involves large myocardial regions, resulting in an appreciable impairment of regional contractility. Conversely, CMD responsible for MVA can be patchily distributed in the myocardium, resulting in sparse small myocardial ischaemic spots that cannot usually determine detectable regional abnormalities in left ventricle contractility.53
However, regional left ventricle dysfunction at echocardiography may also be undetectable in some patients with minor degrees of obstructive CAD,54 while, on the other hand, reversible wall motion abnormalities have been reported in patients with MVA in a few studies.55,56
Radionuclide Stress Tests
Radionuclide stress tests also show similar reversible myocardial perfusion defects caused by CMD or obstructive CAD.57,58 A totally negative myocardial perfusion stress test in the presence of chest pain and ischaemic ST-segment changes might suggest MVA with diffuse CMD. A similar finding, however, may sometimes also be found in patients with multivessel obstructive CAD.59
Advanced Imaging Stress Tests
Stress tests with PET, CMR or myocardial contrast echocardiography can be used to detect abnormalities in myocardial perfusion and ischaemia, but may present similar issues as those described for myocardial scintigraphy. As discussed above, these methods can instead be significant for the assessment of CMD in NO-CAD patients.
Diagnostic Algorithm
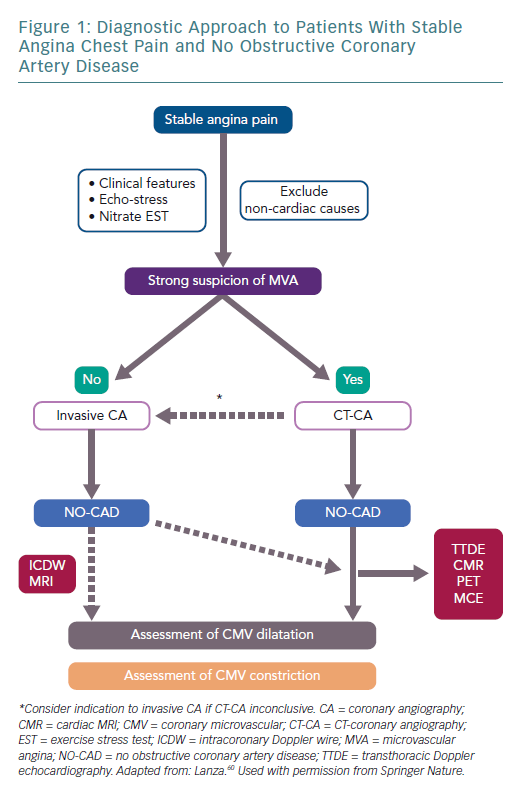
Figure 1 shows a schematic diagnostic approach for patients with a suspicion of primary stable MVA, as well as MVA occurring in other clinical contexts.60 When clinical and non-invasive assessment of the angina patient suggests CMD rather than obstructive CAD, a CT coronary angiography could be recommended to document normal (or near-normal) coronary arteries. The demonstration of CMD might be achieved through a systemic pharmacologic vasodilator test (adenosine/dipyridamole/regadenoson), with the diagnostic method chosen according to the availability and expertise of the single centres.
An ergonovine test might be performed when vasodilator tests are negative or to fully characterise the functional abnormalities of coronary circulation, although with the caveat that the site of vasoconstriction (microvascular or epicardial) cannot be established with certainty.
Invasive coronary angiography, on the other hand, should be directly recommended in angina patients with only a low-to-moderate probability of MVA. Provocative tests should only be performed during the invasive diagnostic procedure in case of detection of normal or near-normal coronary arteries.
Whether careful characterisation of functional abnormalities of coronary circulation (and CMD in particular) by this approach will impact the therapeutic management of patients needs to be ascertained in adequately designed randomised studies.