The publication of the 2022 European Society of Cardiology (ESC) cardio-oncology guidelines provides an important milestone in the cardiovascular care of adult cancer patients and adult survivors of childhood and adolescent cancers.1 The recommendations were developed through a concerted international effort, with collaboration from the ESC, the European Society for Therapeutic Radiology and Oncology, the European Haematology Association and the International Cardio-Oncology Society (IC-OS). The guidelines contain 272 new recommendations and supplant both the 2016 ESC position paper and the 2020 European Society of Medical Oncology guidance on the management of cardiac disease throughout oncological treatment.2,3 Due to the relative novelty of the field and emerging evidence base, the majority of recommendations (208; 76%) carry a Level of Evidence: C rating, i.e. they are supported by expert opinion, small or retrospective studies, or registry data. There are only five (3%) recommendations that carry a Class I, Level of Evidence A recommendation. Overall the guidelines give a broad outline of cardiovascular care before, during and after cancer treatment.
The overarching goals of the guidelines are to ensure baseline cardiovascular (CV) risk is well defined, minimise the risk of cancer therapy-related CV toxicity (CTR-CVT) during and after treatment, provide treatment recommendations for when CTR-CVT occurs, facilitate cancer treatment with minimal unnecessary interruptions, and guide CV surveillance and risk assessment in long-term follow-up after the first year of stopping cancer treatment. CTR-CVT is used as a broad term throughout the document to encompass chemotherapy-related cardiac dysfunction (CTRCD; Table 1), immune checkpoint inhibitor (ICI) myocarditis (Table 2), vascular toxicity, arterial hypertension and cardiac arrhythmias. The guidelines highlight CV risk as a dynamic variable and provide personalised recommendations tailored to the cancer treatment used and pre-existing comorbidities. A multidisciplinary approach is recommended throughout, with involvement predominantly from cardiologists, oncologists and haematologists. While a proportion of cancer patients with cardiac disease can be managed with reference to the ESC guidelines generated for the general population, many cannot, and this reference fills a crucial knowledge gap.4–13 Here, we summarise the important points from this document; however, we encourage interested clinicians to read the guideline document in its entirety.
Baseline Risk Stratification
Many of the recommendations rely heavily on baseline risk assessment as this will determine the trajectory of the future CTR-CVT risk profile, and type, intensity and duration of surveillance. The guidelines provide a checklist for baseline CV toxicity risk assessment that incorporate previous cancer history and treatment, and CV history and risk factors. Further testing should incorporate ECG in all patients, transthoracic echocardiography (TTE) if the patient is at high or very high risk of CTR-CVT and cardiac biomarker measurement (natriuretic peptides [NP], including either B-type natriuretic peptide or N-terminal pro-B-type natriuretic peptide, and cardiac troponin [cTn]) if these markers are to be used to assess dynamic change during follow-up. Other recommended baseline tests include lipid profile, fasting plasma glucose or HbA1c, and kidney function assessment.
Using this information, patients are stratified into different cardiotoxic risk categories. Various risk assessment tools exist; however, the guideline suggests that the Heart Failure Association-International Cardio-Oncology Society (HFA-ICOS) risk scoring system should be used.1,14 The HFA-ICOS is a comprehensive tool that includes previous CV disease, imaging, biomarkers, age, general comorbidities, previous cancer treatment and lifestyle factors; the variables have different weighting depending on the proposed cancer treatment. Following stratification, cardiology referral is recommended in patients deemed to be at high and very high risk of CTR-CVT, and in patients with pre-existing CV disease or abnormal baseline cardiac investigations prior to cancer therapy. All low-risk patients should proceed to cancer therapy without delay.
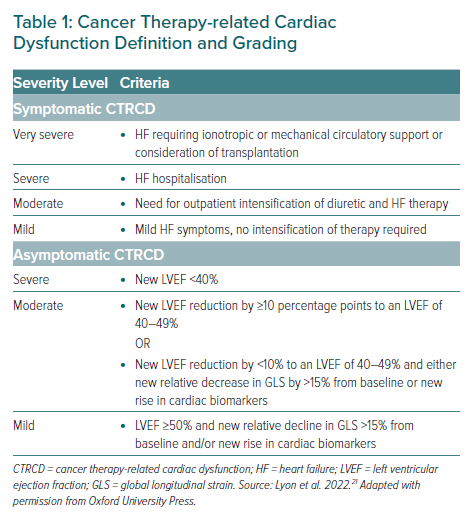
Due to the multidisciplinary nature of cardio-oncology, there is a wide range of disease definitions that originate from different clinical specialisms. The 2022 ESC cardio-oncology guidelines use the standardised CTR-CVT definitions created by IC-OS.15 These diagnostic categories provide harmonisation where many definitions can exist for a single CTR-CVT (such as the definitions of cancer therapy-related cardiac dysfunction or hypertension).
Prevention and Monitoring During Therapy and the First 12 Months After the End of Therapy
The risk of CTR-CVT is dependent on many variables and the guidelines highlight that CV disease prevention prior to cancer therapy should follow established ESC guidance.11 Patients should have modifiable risk factors addressed and although exercise is beneficial for cardiorespiratory fitness, this is mentioned with the caveat that there is no clear benefit of performing exercise before or during cancer treatment to reduce CTRCD.16
Specific primary CV disease prevention guidance is given for patients at high and very high risk of CV toxicity with selected treatments. Dexrazoxane or liposomal anthracyclines should be considered alongside treatment with angiotensin-converting enzyme inhibitors (ACE-i) or angiotensin receptor blockers (ARB), β-blockers and statins to prevent CTRCD in patients receiving anthracyclines. ACE-i/ARB, β-blockers and statins should be considered in patients receiving human epidermal receptor 2 (HER2)-targeted therapies or those receiving cancer therapy associated with heart failure.
Primary prevention of radiotherapy-induced CV toxicity is discussed; due to the late vascular toxicity with accelerated coronary artery disease in this cohort, stringent cardiovascular risk factor (CVRF) control is required. For quantitating radiation exposure, the mean heart dose (MHD) is used in preference to the prescribed dose as it more accurately reflects cardiac radiation exposure; however, there is flexibility within this grading and patients who receive a relatively high dose to a small area of the heart may be at lower CV risk than that dictated by the MHD. Most of the contemporary advances in mitigating radiation-induced cardiotoxicity have occurred through technological developments that improve targeting of the therapy and reduce the MHD.
General secondary prevention strategies for patients prior to cancer treatment should follow previous ESC guidance. The new guidelines provide comprehensive therapy-specific screening protocols to facilitate CTR-CVT detection with subsequent treatment and secondary prevention of cardiovascular disease if it occurs during cancer therapy (Supplementary Material Table 1). The screening protocols cover the first 12 months after treatment in order to detect sub-acute CTR-CVT. The evidence supporting this follow-up time window was extrapolated from a study of patients receiving anthracycline therapy, of whom 98% of the cases of cardiotoxicity were detected within the first 12 months after treatment.17
In addition to the screening protocols, cyclophosphamide, platinum-containing therapy, arsenic trioxide, and FMS-like tyrosine kinase 3 inhibitor CTR-CVT’s are discussed; these agents do not have specific screening protocols because their toxicity is either relatively acute or poorly defined.
Acute and Subacute Toxicity
A proportion of patients with cancer who develop CV disease can be managed according to general ESC guidance; however, patients may have several factors that necessitate deviation from accepted standards. For example, the antiplatelet strategy in acute coronary syndrome (ACS) may differ from standard practice in a thrombocytopaenic patient on active chemotherapy with curable intent.
In general, the new guidelines recommend that all patients who develop CTR-CVT should be discussed at a multidisciplinary team (MDT) meeting and reviewed either through a specialised cardio-oncology service, or by a cardiologist with expertise in managing CV disease in cancer patients. Important factors to consider include cardiac and cancer disease burden, cancer prognosis, available options for alternative less cardiotoxic cancer regimens, drug–drug interactions (DDI) and patient preferences. Recommendations for the management of specific toxicities presented in the guidelines are summarised below.
Anthracycline Cancer Therapy-related Cardiac Dysfunction
The guidelines stratify management by symptomatic and asymptomatic cancer therapy-related cardiac dysfunction (CTRCD, as per the IC-OS CTR-CVT definitions), with recommendations differing between the groups depending on the severity of CTRCD. For symptomatic patients, heart failure therapy should be started following standard ESC guidance for heart failure treatment, with the decision to continue or stop anthracyclines dependent on the severity of symptoms.9 For asymptomatic patients with moderate or severe CTRCD, treatment should be interrupted, whereas those with mild disease should continue treatment with the introduction of ACEi/ARB and/or β-blockers if certain criteria are met. Liposomal anthracycline and dexrazoxane may be used as secondary prevention strategies in situations where anthracyclines are restarted after the development of moderate or severe CTRCD.
HER2-Targeted Cancer Therapy-related Cardiac Dysfunction
Like the anthracycline guidance, HER2-targeted CTRCD management is also stratified by the presence or absence of symptoms. The guidelines provide a more lenient approach to the continuation of therapy in this cohort, reflecting a higher likelihood of left ventricle (LV) functional recovery. Symptomatic patients with moderate to severe CTRCD should have HER2- treatment interrupted, and heart failure therapy started; the decision to restart or stop HER2-targeted treatment should be made by an MDT. Patients with mild symptomatic CTRCD should have heart failure treatment started and a multidisciplinary consensus on whether to continue or interrupt therapy. For asymptomatic patients, those with moderate and mild disease can continue HER2 treatment with increased monitoring (TTE, cTn and NP) and consideration of ACEi/ARB and β-blockers.
Immune Checkpoint Inhibitor-associated Cardiovascular Complications
Immune checkpoint inhibitors (ICIs) are associated with a range of direct and indirect CV toxicities. Direct effects include myocarditis, atrial and ventricular tachyarrhythmias and bradyarrhythmias, ACS, stroke, pericardial inflammation, dyslipidaemia, takotsubo syndrome and non-inflammatory LV dysfunction. Indirect effects manifest through inflammation of the thyroid, adrenal and pituitary glands, and pancreas, alongside vascular changes leading to an increased risk of arterial and venous thrombosis.
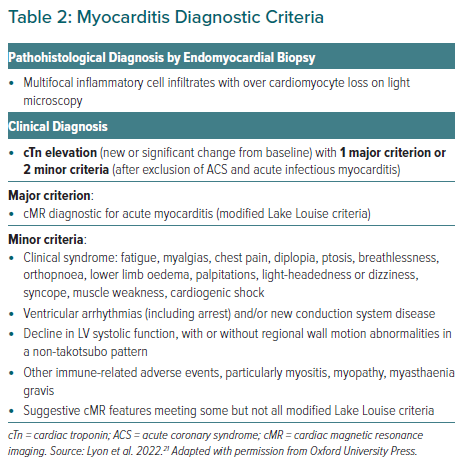
Although rare, when ICI-associated myocarditis occurs, the mortality rate is high. The diagnosis should be suspected in patients with CV symptoms or non-cardiac immune-related adverse events (particularly myasthenia, myositis and myopathy) associated with a troponin elevation and new ECG changes. Specific diagnostic criteria exist (Table 2); however, the guidelines highlight the practical reality that treatment may need to be instigated before these are fulfilled. Urgent cardiac imaging and assessment for other aetiologies of myocardial injury (predominantly ACS or viral myocarditis) is required. Intravenous (IV) methylprednisolone should be commenced before confirmatory testing in haemodynamically unstable patients and ICI therapy should be interrupted. TTE and cardiac magnetic resonance imaging (cMRI) are recommended to aid diagnosis, although modified Lake Louise criteria are used for cMRI classification.18 Cardiac fluorodeoxyglucose PET is another imaging option; however, it is difficult to perform a strict carbohydrate fast in these patients. Endomyocardial biopsy is recommended where suspicion persists, and the imaging and biomarkers are equivocal.
The guidelines classify myocarditis as fulminant (haemodynamically unstable with heart failure requiring ventilatory support and/or either high-grade atrioventricular [AV] block or ventricular arrhythmias) or non-fulminant (symptomatic but stable and incidental cases). The main practical implication of this is that unstable patients should be monitored in a level 3 environment that can deliver mechanical circulatory support, if required. CV complications should be managed as per specific ESC guidance.5,9,12,13 Other patients should receive continuous cardiac monitoring to detect new atrioventricular (AV) blocks or tachyarrhythmias.
Both fulminant and non-fulminant patients should receive IV methylprednisolone 500–1000 mg given daily for 3–5 days. This should be initiated promptly to reduce major adverse cardiovascular outcomes and mortality. Although the guidelines suggest a single dose of methylprednisolone should be given in suspected cases, there may be situations where steroids need to be continued for longer until the diagnosis is confirmed, e.g. due to ongoing haemodynamic instability precluding cMRI. Switching to oral prednisolone can occur if there is clinical improvement within the first 24–72 hours, defined as symptom resolution, recovery of LV function, restoration of baseline rhythm and >50% cTn decline. If there is no clinical improvement within 72 hours of therapy, then the patient is steroid-resistant. There is no consensus on either the optimal steroid reducing or secondary immunosuppression regimens. Any decision to recommence ICI after recovery should be discussed through an MDT.
Chimeric Antigen Receptor T-cell and Tumour-Infiltrating Lymphocytes Therapies and Heart Dysfunction
The recommendations for chimeric antigen receptor T (CAR-T) and tumour-infiltrating lymphocytes (TIL) CTRCD management derive from smaller studies and case reports.19 Most adverse events with CAR-T therapy are linked to cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome. The most common CV complications are arrhythmias, heart failure, myocardial infarction and venous thromboembolism. When cardiotoxicity occurs, patients require ECG monitoring, TTE, cTn and NP assessment. The potential for malignant arrhythmias and multi-organ failure necessitates level 3 care. Interleukin-6 elevation supports the diagnosis of CRS with tocilizumab and steroids providing treatment options. Cardiac complications with TIL, while common, do not appear to affect survival and the guideline does not have any specific recommendations for CV management in these patients beyond standard ESC guidance.
Haematopoietic Stem Cell Transplant-induced Cardiovascular Disease
A wide range of CV complications can occur during haematopoietic stem cell transplant (HSCT), which, although rare, are clinically significant. There is a relative lack of evidence for interventions to limit CV toxicity in this cohort and the guidelines call for further studies within this area, including the role of exercise before and after transplant.
Takotsubo Syndrome
There are numerous aetiological factors responsible for takotsubo syndrome (TTS) in cancer patients, including treatments delivered and the emotional stress associated with the diagnosis and preceding investigations. As per management in the non-cancer cohort, the guideline recommends angiography with CT or invasive coronary angiography, alongside cMRI to look for evidence of infarct or myocarditis. If a medication is thought to be causative (e.g. 5-fluorouracil), then this should be held during the acute phase alongside any QT prolonging drugs, with an MDT discussion regarding restarting the treatment. In the situation of immune checkpoint inhibitor TTS, steroids are recommended if there is inflammation in a typical TTS pattern on cMRI.
Coronary Artery Disease
Management recommendations are given for both ACS and chronic coronary syndromes (CCS), with the guidelines acknowledging that most of the evidence is based on observational and registry data. Cancer patients are at an increased risk of ACS compared to the general population and alongside atherosclerotic plaque rupture, both vasospasm (such as that induced by fluoropyrimidines) and coronary thrombosis are prevalent aetiologies in this cohort. The diagnostic work-up is the same as in the non-cancer patient, although the presentations may be atypical, lending greater weight to TTE assessment in the initial management.
The guidelines highlight evidence that cancer patients have invasive coronary management less frequently than those without cancer, and where it is used, percutaneous coronary intervention (PCI) is associated with a reduction in adverse outcomes and all-cause mortality. The exception to this is in a cohort of patients with non-ST-segment elevation myocardial infarction (NSTEMI) and advanced cancer where a mortality benefit of PCI over medical therapy has not been shown. Given these findings, an early invasive strategy is recommended for patients with cancer and ST-segment elevation myocardial infarction (STEMI) or high-risk NSTEMI where life expectancy is greater than 6 months. PCI can also be considered as a palliative option to treat ACS complications, such as pulmonary oedema and ventricular tachyarrhythmias refractory to medical therapy. Where patients have STEMI or NSTEMI in the context of poor cancer prognosis (less than 6 months) and/or a very high bleeding risk, then a non-invasive strategy should be pursued.
Where stenting is indicated, third-generation drug-eluting stents should be used. Operators should use fractional flow reserve or instantaneous wave-free ratio and imaging with intravascular ultrasound and optical coherence tomography to ensure intervention is indicated and thrombotic complications are reduced. Post-procedure, dual antiplatelet therapy (DAPT) duration should be as short as possible (1–3 months), ideally with the use of aspirin and clopidogrel. Where anticoagulation is indicated, triple therapy should not last longer than 1 week, ideally given while the patient is in hospital. Patients may be suitable for coronary artery bypass grafting (CABG) if their cancer prognosis is greater than 1 year.
Specific recommendations are provided for thrombocytopenic patients. PCI and CABG are not advised if the platelet count is <30,000 and <50,000/μl, respectively, although diagnostic angiography can be undertaken with radial access and close attention to haemostasis, adjusted heparin dosing and platelet transfusion beforehand if <20,000/μl. Aspirin should be avoided with a platelet count <10,000/μl, clopidogrel should be avoided with a count <30,000/μl, and prasugrel and ticagrelor avoided with a count <50,000/μl. In the case of fluoropyrimidine-induced vasospasm, a rechallenge can occur in a monitored environment if there is no alternative treatment, severe coronary disease is excluded, and prophylactic medication (nitrites, calcium channel blockers) are commenced. Cancer treatment may need to be stopped or altered and all these patients’ ongoing treatment should be planned through an MDT, starting with urgent multidisciplinary discussion at the time of ACS diagnosis.
The management of patients with chronic coronary syndromes is similar to those without cancer; however, interventional treatment decisions in this context need to be planned by an appropriate MDT. The risk of acute and late complications (in particular, bleeding) is increased in this cohort and DAPT duration should be individualised to the patient and kept as short as possible.
Valvular Heart Disease
Two main situations are addressed in the guideline: patients who have pre-existing severe valvular heart disease, including those identified at baseline assessment and those who have severe valvular heart disease that develops during cancer therapy. In both situations, patients should be managed in accordance with the ESC/European Association for Cardio-Thoracic Surgery guidelines, and an MDT is required to determine the optimal type of valve treatment.10 Important factors impacting treatment include cancer-related prognosis, technical feasibility of surgery, urgency of cancer treatment, and the presence of coexisting conditions such as endocarditis.
Cardiac Arrhythmias
The guidelines address the management of AF, ventricular arrhythmias, long corrected QT intervals, and bradyarrhythmias. The recommendations will be summarised in this order. There is an appreciation of additional pathophysiological factors for AF development in cancer patients, including increased inflammation, paraneoplastic manifestations, localised cancer invasion, the effect of surgery, and increased AF incidence with specific cancer treatments (e.g. ibrutinib). The choice between rate or rhythm control is more complex as pharmacological rhythm control is less likely to be successful (with an increased likelihood of QT prolongation) in the presence of cancer with an increased risk of drug interactions, hence rate control with β-blockers initially may be more appropriate unless there is haemodynamic instability.
The guidelines recommend that anticoagulation decisions use an integrative model which incorporates the assessment of thromboembolic risk, bleeding risk, drug-drug interactions, and patient preferences and drug availability (TBIP). The CHA2DS2-VASc score should be used for thromboembolic risk stratification, while acknowledging it may under-estimate thrombotic risk; this is reflected in the recommendation that patients with cancer, AF and CHA2DS2-VASc 0 (men) and 1 (women) may be considered for therapeutic anticoagulation after the bleeding risk is considered. Bleeding risk assessment is extended beyond tools such as HAS-BLED to include cancer-specific factors; specifically, the presence of thrombocytopenia, gastrointestinal or genitourinary malignancy, active or recent bleeding and recent or evolving intracranial lesions.20 As in the non-cancer cohort, vitamin K antagonists should be used in patients with mechanical heart valves and moderate-to-severe mitral stenosis. For all other patients, direct oral anticoagulants (DOACs) are preferred with low-molecular weight heparin (LWMH) being a second choice and left atrial appendage occlusion reserved for patients unsuitable for anticoagulants who have a life expectancy greater than 12 months.
Most ventricular arrhythmias originate from QTc prolongation leading to torsades de pointes (TdP). The risk of TdP increases two- to three-fold with a QTc ≥500 ms, with TdP being rare if the QTc <500 ms.12 The Fredericia formula is recommended for QTc calculation due to better performance at high and low heart rates. Different cancer therapies are associated with different risks of QTc prolongation and when TdP or sustained ventricular tachyarrhythmias occur, the associated cancer drug should be stopped. When the QTc ≥500 ms in the absence of ventricular arrhythmias, the causative drug should be stopped, reversible factors such as electrolyte abnormalities corrected, and daily ECG monitoring should occur until resolution of the QTc to baseline. Patients with a QTc 480–500 ms should have weekly ECG monitoring while on treatment. If a decision to restart QTc- prolonging therapy after temporary interruption has been made, weekly ECGs for the first 4–6 weeks and then monthly are recommended. Given the increased life expectancy, especially for those living longer than 1 year after diagnosis, more patients are becoming eligible for ICDs or catheter ablation in the correct clinical context after MDT discussion.
Bradyarrhythmias directly related to cancer treatment are less common. In the context of ICI-induced AV conduction disease, the guidelines recommend serial ECG monitoring with hospitalisation if the PR extends to >300 ms and the initiation of IV methylprednisolone. Sinus bradycardia is a side-effect of immuno-modulatory drugs and anaplastic lymphoma kinase (ALK) inhibitors; the management of patients with significant symptoms (syncope, pre-syncope) requires an MDT approach to decide on the cancer treatment strategy and indication for pacing.
Arterial Hypertension
As in other areas of the guidelines, the goal of hypertension treatment is to prevent unnecessary interruptions to cancer therapy and reduce CV complications. Arterial hypertension can be induced directly by treatment (e.g. ibrutinib, vascular endothelial growth factor inhibitors), indirectly (e.g. corticosteroid use) and through factors such as pain and obesity. Treatment threshold recommendations are presented and take into consideration the cancer prognosis, with less stringent treatment thresholds in patients with metastatic cancer and reduced life expectancy. For patients with curable cancer during treatment, a target of <140 mmHg systolic and <90 mmHg diastolic is recommended, with the consideration of more stringent control (<130/80 mmHg) if treatment is well tolerated. In the metastatic setting, a blood pressure under 140–160 mmHg systolic and <90–100 mmHg diastolic can be considered as an optimal target.
ACEi/ARB are recommended first-line agents, with dihydropyridine calcium channel blockers (CCB) second line; there is no recommended age or ethnicity-specific alteration in first-line treatment. Where the blood pressure is ≥160 mmHg systolic and ≥100 mmHg diastolic, combined ACEi/ARB and CCB are recommended alongside withholding the hypertension-associated cancer therapy. Non-dihydropyridine CCB should be avoided due to the potential for DDI.
Thrombosis and Thromboembolism
The guidelines present the management of venous thrombosis and thromboembolism (VTE) in a similar way to the management of anticoagulation in AF using the TBIP model. VTE is significantly more common in cancer patients, and it is worth noting that the recommendations for the use of low molecular weight heparin (LMWH) as anticoagulation in patients with cancer and AF are stratified from the results of studies on VTE treatment in cancer patients. Both DOACs (apixaban, edoxaban or rivaroxaban) and LMWH carry level IA recommendations for the treatment of VTE in patients receiving cancer treatment; however, certain situations (DDIs, severe renal dysfunction, unoperated gastrointestinal or genitourinary cancer) favour LMWH use over DOACs. The specific acute management of VTE presentations will depend on the initial site of the thrombus (e.g. peripheral venous, pulmonary, intracardiac).
For primary prevention, hospitalised or prolonged bed-rest patients should receive prophylactic LMWH and patients with major abdominal or pelvic surgery should receive extended prophylaxis with LMWH for 4 weeks postoperatively. There may be a role for primary prevention in select high-risk ambulatory patients; however, the guidelines note more research is needed in this area.
Other Acute Management
To round off the comprehensive acute management section, guidance is given for peripheral arterial disease, pulmonary hypertension (PH) and pericardial disease. All types of PH can present in cancer patients; however, the tyrosine kinase inhibitor dasatinib is strongly associated with PH. In cases where the tricuspid regurgitant velocity (TRV) is >3.4 m/s, dasatinib should be withheld and the diagnosis confirmed with right heart catheterisation. An alternate breakpoint cluster region-abelson (BCR-ABL) inhibitor should be introduced when the TRV <2.7 m/s.
In general, the management of pericarditis follows established ESC guidance.4 Stable patients with small to medium sized cancer-associated pericardial effusions (>4 to <20 mm) should have reassessment 7–14 days after diagnosis and then every 4–6 weeks unless intervention is required at the time of initial reassessment. Where pericarditis is associated with ICI, multimodality imaging, ECG and biomarker assessment are required to investigate disease severity and evidence of concomitant myocarditis. If the effusion is moderate to severe, the ICI should be withheld, and colchicine and prednisolone should be used to reduce the inflammation. A multidisciplinary meeting is needed to decide whether to restart the ICI.
End of Therapy Assessment and Long-term Follow-up
The guidance separates risk assessment within the first 12 months following treatment and ongoing risk assessment and surveillance beyond the initial 12 months and encompasses both adult and childhood and adolescent cancer survivors. These recommendations are only relevant to patients who have a good long-term prognosis. Patients are stratified by both early and late risk, and symptomatology into very high, high, moderate and low-risk groups. Early high risk comprises those with high or very high pre-treatment risk (HFA-ICOS assessed), recipients of therapies associated with a long-term risk of complications, patients developing CTR-CVT during treatment, and/or patients identified to have new abnormalities on TTE, biomarkers or symptoms at the end-of-therapy assessments. Interested readers should refer to the guidelines for description of the risk categories.
Therapies associated with long-term risk of CV complications include doses of doxorubicin ≥250 mg/m2 (or equivalent), radiotherapy >15 Gy mean heart dose (MHD), doxorubicin ≥100 mg/m2 combined with radiotherapy 5–15 Gy MHD, and high-risk haematopoietic stem cell transplant recipients. Educating patients regarding healthy lifestyle choices, their risk of disease, and cardinal CV symptoms to watch for is recommended; this is especially important in childhood and adolescent survivors of cancer therapies where CTRCD is a significant contributor to morbidity and mortality later in life. From 12 months, annual screening and modifiable CV risk factor management are recommended. Adult patients should have risk re-stratification at 5 years post-therapy and all patients with emergent cardiovascular symptoms should have a cardiology assessment.
Regarding imaging, adult survivors at moderate risk should have a TTE every 5 years and those at high or very high risk should have a TTE 1, 3 and 5 years after therapy. Childhood and adolescent survivors should have a TTE every 5 years if they are at moderate risk, and every 2 years if high or very high risk.
An additional mention is made of renal artery and carotid disease; renal artery ultrasound is recommended for those who received abdominal and pelvic radiation and who have worsening renal function and/or hypertension, and carotid ultrasound imaging is recommended for asymptomatic patients with head and neck radiotherapy starting 5 years after treatment and continued for every 5–10 years. Long-term follow-up strategies for specific diseases (coronary artery, valvular, pericardial, stroke, peripheral arterial, arrhythmias, autonomic) are presented. This section focuses mostly on the long-term sequelae of radiotherapy but also discusses other associated risks, including platinum-based therapies and coronary artery disease, and nilotinib and peripheral arterial disease. The management of metabolic disorders, pregnancy, and pulmonary hypertension in cancer survivors is also covered in this section.
Special Populations
The final section covers primary cardiac tumours, cardiac assessment of pregnant patients with cancer, carcinoid heart disease, amyloid light chain cardiac amyloidosis, and cardiac implantable electronic devices (CIED). The CIED section focuses on the interaction of radiotherapy and device function, with a consideration of the interaction between the type of radiotherapy (in particular, the level of neutron emission exposure), treatment volume, and whether the patient is pacing dependent or has regular therapies if they have an ICD in situ.
Conclusion
The new ESC cardio-oncology guidelines provide the cornerstone for recommendations to specialist and general cardiologists treating cancer patients and represent a remarkable achievement considering the breadth and depth of the document in a relatively novel field. There are, however, numerous points to be addressed in the future to ensure many of the recommendations have a stronger evidence base. The authors acknowledge this and state many future directions for training, service provision and research, including the need for training pathways encompassing a multidisciplinary approach, studies to validate the HFA-ICOS risk assessment tools, and assessment of the impact of genetic susceptibility to cardiotoxicity with certain cancer treatments.
From a practical perspective, there is a need to better understand the cardiovascular impact of treatment combinations (beyond anthracyclines and radiotherapy), define the cost-effectiveness of screening and surveillance strategies and their impact on long-term health outcomes in cancer patients, and ascertain how this resource-heavy approach will be practically implemented within healthcare systems. Through outlining the known unknowns in the final sections of the document, the future road map for cardio-oncology has been established, providing fertile ground for expansion of the subspeciality.