Chronic coronary syndromes (CCS) have been defined as a clinical presentation of coronary artery disease (CAD), encompassing all evolutionary phases except episodes wherein acute thrombosis predominates, which constitutes acute coronary syndromes (ACS).1 However, the transition from ACS to CCS has not been well-defined.
Certain conditions may increase the risk for future cardiovascular events in CCS.1 Old age, co-morbidities such as diabetes and chronic kidney disease (CKD), and complex CAD have been established as risk factors for ischaemia.2–9 On the other hand, some of these conditions may also affect bleeding risk from antiplatelet therapy. Advanced age, diabetes and CKD have been found to confer an increased risk for haemorrhagic events among patients taking antiplatelet agents for CAD or after percutaneous coronary intervention (PCI).10–23
Consequently, for such specific populations, modification of usual therapy may be warranted. European guidelines have recently suggested careful consideration of dual antiplatelet therapy (DAPT) for these patient subgroups.1 DAPT with aspirin plus a P2Y12 inhibitor has already been established as a mainstay preventive therapy after ACS and/or PCI.24–26 Meanwhile recommendations on its use for special populations with CCS, and their applicability to Asian patients, have yet to be well elaborated.
A number of characteristics may limit transferability of results from predominantly Western trial populations to Asian patients. These include – but are not limited to – reduced bioactivation of certain drugs (i.e. clopidogrel) from genetic polymorphisms, a lower risk of ischaemic events and higher bleeding risk among East Asians undergoing stent implantation, a higher risk of major bleeding among patients with ACS, a high rate of stroke and low rate of cardiovascular death or non-fatal MI among patients with CCS, and a higher prevalence of diabetes among CAD patients – particularly in the Middle East – and local differences in clinical practice.27–31
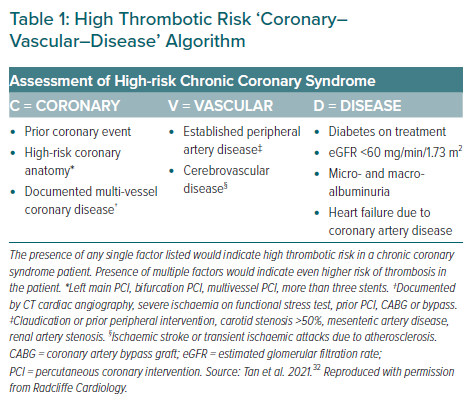
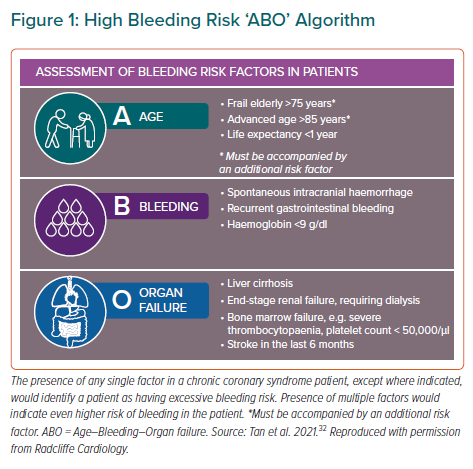
Clopidogrel and the more potent agents ticagrelor and prasugrel constitute the P2Y12 inhibitors in current practice. Previously, we had provided recommendations on the use of these agents for Asian patients with ACS, including general statements for special populations.26 We subsequently produced a consensus paper on the management of high-risk CCS, where we proposed two sets of criteria: the Coronary–Vascular–Disease criteria, distinguishing high-risk from very-high risk CCS (Table 1); and the Age–Bleeding–Organ failure (ABO) criteria, describing bleeding risk among Asian patients with CCS (Figure 1).32
In this consensus paper, we aimed to summarise key evidence and present recommendations on the use of P2Y12 inhibitors, with a focus on newer-generation P2Y12 inhibitors, for CCS among special populations in Asia. These include those transitioning from ACS to CCS, those with CKD, the elderly, those with diabetes, those with multivessel CAD and those who bled with the use of antiplatelet therapy.
Methods
The Asian Pacific Society of Cardiology (APSC) convened a panel of experts from various regions and countries in Asia with clinical and research expertise in P2Y12 inhibitors, to develop consensus statements regarding the use of these drugs among special populations in Asia. The experts were members of the APSC who were nominated by national societies and endorsed by the APSC consensus board.
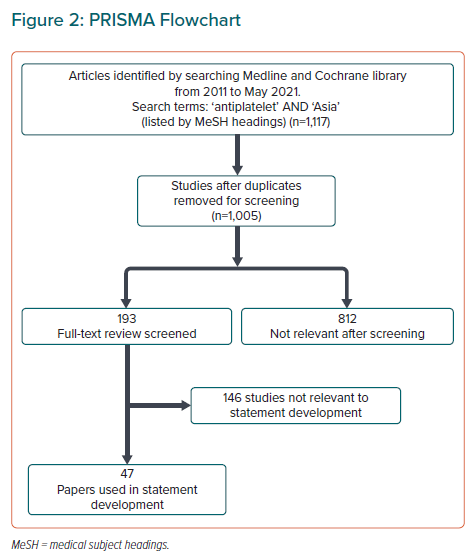
We conducted a comprehensive literature search (Figure 2), with preference for randomised trials that evaluated the efficacy (in terms of ischaemic outcomes) and safety (in terms of haemorrhagic outcomes) of antiplatelet regimens containing the newer-generation P2Y12 inhibitors, among patients with CAD. Relevant articles were reviewed and appraised for quality and risk of bias using the Grading of Recommendations Assessment, Development, and Evaluation system, as follows:33
- High (authors have high confidence that the true effect is similar to the estimated effect).
- Moderate (authors believe that the true effect is probably close to the estimated effect).
- Low (true effect might be markedly different from the estimated effect).
- Very low (true effect is probably markedly different from the estimated effect).
The collected relevant body of evidence was then extracted, presented, and discussed during two consensus meetings, during which consensus statements were constructed. Each statement was subsequently put to an online vote, where every panel member voted using a three-point scale (agree, neutral or disagree). Consensus was reached when 80% of votes for a statement were agree or neutral. In case of non-consensus, the statements were further discussed via email communication and revised accordingly until consensus was reached.
Consensus Statements
Single Antiplatelet Therapy in Patients Transitioning from Acute Coronary Syndrome To Chronic Coronary Syndrome
In the aforementioned APSC consensus statement on the management of high-risk CCS, the APSC recommended various antithrombotic management strategies based on the patient’s ischaemic and bleeding risk.32 However, since its development, several studies and guidelines have been published, which warranted a review of the treatment of patients with CCS, particularly those transitioning from ACS to CCS.
The 2020 European Society of Cardiology (ESC) guidelines on non-ST-elevation MI recommended the use of various regimens of shortened DAPT in selected patients, based on four trials: TWILIGHT, GLOBAL LEADERS, SMART-CHOICE, and SMART-DATE.34–37 For our consensus statements, the primary references were TWILIGHT, SMART-CHOICE, and two other Asian trials: STOPDAPT-2 and HOST-EXAM.34,36,38,39
TWILIGHT (NCT02270242) was a double-blind, randomised trial that involved 7,119 adult subjects from North America, Europe, and Asia who underwent successful PCI.34,40 While all patients enrolled in the trial were deemed by the investigators to be at high risk for either ischaemia or bleeding, only a few of the subjects would be considered at high bleeding risk if the APSC ‘ABO’ criteria were used.40 Almost a quarter (~23%) of patients were Asian, compared with ~1% in GLOBAL LEADERS.34,41 More than half of the subjects (~65%) had ACS at presentation.34 After 3 months of treatment with ticagrelor plus aspirin, patients who had not had a major bleeding event or ischaemic event continued to take ticagrelor and were randomly assigned to continue aspirin or receive placebo for 1 year.34 Patients in the single antiplatelet therapy (SAPT) arm experienced a lower risk of bleeding than those in the DAPT arm.34 In contrast, there was no significant difference in ischaemic outcomes between the two groups.34
SMART-CHOICE (NCT02079194) was an open-label, single-blind, randomised trial that studied 2,993 adult patients from Korea who had undergone successful PCI for either CCS (~42%) or ACS (~58%).42 All subjects underwent 3 months of DAPT and were then randomly assigned to receive either SAPT with a P2Y12 inhibitor or continuous DAPT for 9 more months.42 The P2Y12 inhibitor used in the regimen, subject to the investigator’s discretion, was any of the three following agents: clopidogrel (77.2%), ticagrelor (18.4%), and prasugrel (4.3%).36 The study found SAPT to be non-inferior to DAPT in terms of ischaemic and bleeding outcomes.36
STOPDAPT-2 (NCT02619760) was an open-label, randomised trial conducted among 3,009 Japanese subjects who had undergone successful PCI.38 Compared to the previous two trials, this study enrolled a smaller population presenting as ACS (~38%).38 After PCI, all subjects received 1 month of DAPT using aspirin plus clopidogrel or prasugrel.38 The experimental group discontinued aspirin after 1 month and shifted to clopidogrel monotherapy while the control group continued DAPT with aspirin plus clopidogrel for 1 year.38 The study revealed that SAPT was superior to DAPT in terms of bleeding outcomes and non-inferior to DAPT in terms of ischaemic outcomes.38
HOST-EXAM (NCT02044250) was an open-label, single-blind, randomised trial conducted in South Korea among 5,438 adult participants.39 Prior to enrolment, the subjects had undergone PCI for either ACS (~72%) or CCS (~28%) and had already been taking DAPT using aspirin and a P2Y12 inhibitor (clopidogrel ~82%, ticagrelor ~10%, prasugrel ~8%) for 6–18 months, without any ischaemic or haemorrhagic complication.39 They were then randomly assigned to receive either clopidogrel or aspirin monotherapy for 24 months.39 In this study, subjects in the clopidogrel group were found to have a significantly lower risk of thrombotic and haemorrhagic events compared to the aspirin group.39
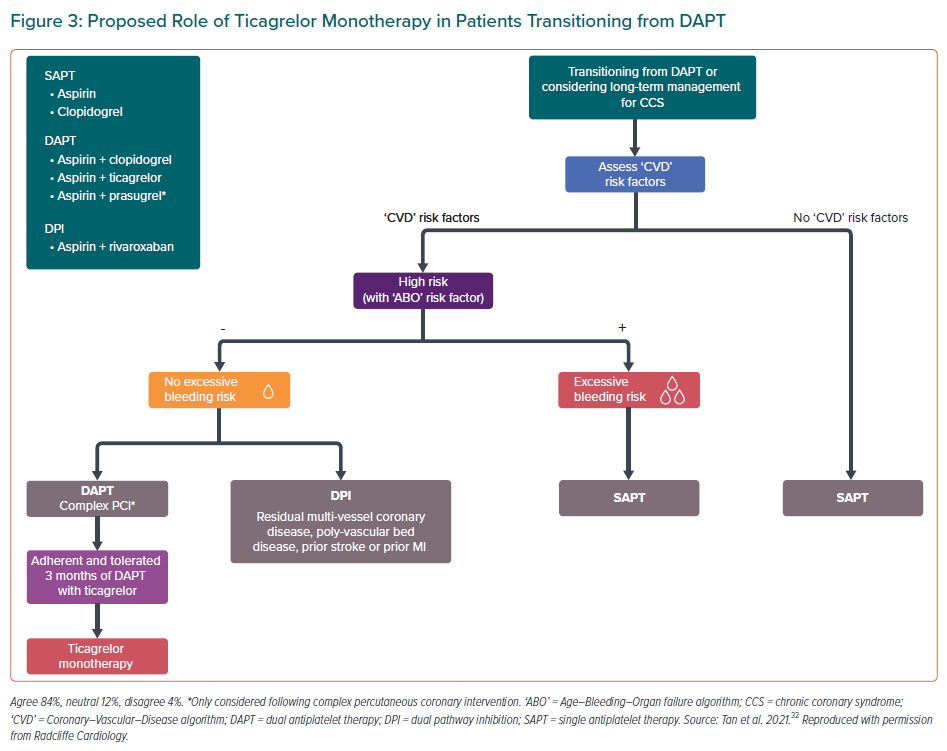
The open-label design of the three Asian trials conferred a risk for performance bias. Nevertheless, given the results of these trials, the consensus panel concluded that the use of ticagrelor monotherapy was reasonable among patients with high ischaemic risk, low bleeding risk and good adherence to 3 months of ticagrelor-based DAPT (Figure 3). On the other hand, based on data from SMART-CHOICE, STOPDAPT-2, and HOST-EXAM, clopidogrel monotherapy may be used for patients with low ischaemic risk or patients with high ischaemic risk and excessive bleeding risk.
Chronic Kidney Disease
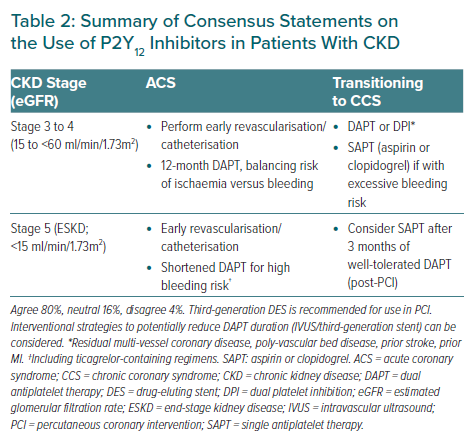
Most of the statements on patients with CKD pertain to the setting of ACS where ischaemic risk is highest.
Statement 1. CKD patients should be assessed for bleeding risk before P2Y12 inhibitor initiation.
Level of evidence: High.
Level of agreement: Agree 85%, neutral 15%, disagree 0%.
Statement 2. Patients with estimated glomerular filtration rate (eGFR) of 15 to 60 ml/min/1.73m2(stage 3A [moderate] to stage 4 [severe]) with previous major adverse cardiovascular event and without excessive bleeding risk should receive DAPT for ACS.
Level of evidence: Moderate.
Level of agreement: Agree 85%, neutral 11%, disagree 4%.
Statement 3. CKD patients with eGFR of <60 ml/min/1.73m2 with excessive bleeding risk may alternatively receive SAPT (aspirin or clopidogrel)
Level of evidence: Moderate.
Level of agreement: Agree 81%, neutral 11%, disagree 8%.
Statement 4. For patients with end-stage renal failure on dialysis with ACS, a shortened duration of DAPT (including ticagrelorcontaining regimens) can be considered.
Level of evidence: Low.
Level of agreement: Agree 88%, neutral 8%, disagree 4%.
Statement 5. Third-generation drug-eluting stent (DES) is recommended for use in PCI for patients with CKD
Level of evidence: Low.
Level of agreement: Agree 92%, neutral 8%, disagree 0%.
Statement 6. Interventional strategies that potentially reduce ischaemic risk (intravascular ultrasound/optical coherence tomography/third-generation DES) can be considered.
Level of evidence: Low.
Level of agreement: Agree 81%, neutral 11%, disagree 8%.
Propensity for bleeding in CKD may arise from quantitative defects (e.g. platelet consumption, redistribution, etc.) or qualitative dysfunction (blunted activation, weakened platelet-vessel wall interactions, etc.).43–48 Meanwhile high thrombopoietin levels, juvenile platelets, and thrombin receptor overactivation in CKD and dialysis may lead to a pro-thrombotic state.49,50 Clinically, post-PCI patients with CKD (eGFR <60 ml/min/1.73m2) have significantly higher rates of ischaemic and bleeding outcomes compared to non-CKD patients.51 This increased ischaemic risk among CKD patients supports the use of DAPT in those with ACS as well as those transitioning to CCS.51,52 A recent systematic review and meta-analysis also suggests that the newer P2Y12 inhibitors are plausible treatment alternatives in CKD patients who may have reduced clopidogrel response.53 A single-centre, prospective, randomised study on patients with CKD and non-ST elevation-ACS also found that ticagrelor provided more potent platelet inhibition compared with clopidogrel in these patients.54
However, before initiating DAPT, risks and benefits must be carefully weighed by the healthcare professional. While the majority of the expert panel agree to the use of SAPT in patients with high bleeding risk, a few experts recommended a shortened DAPT regimen prior to SAPT.
Published data on CAD patients with end-stage kidney disease (ESKD) or on dialysis have remained scarce.1 The European CCS guidelines allude to the presence of CKD with eGFR 15–59 ml/min/1.73m2 as conferring a moderate risk for ischaemia while the presence of ESKD confers a high bleeding risk.1 In a Korean retrospective cohort study among post-stenting patients on dialysis – composed mostly of subjects with ACS (>70%) – prolonged DAPT >12 months reduced the risk of major adverse cardiovascular events but tended to correlate with a higher probability of bleeding compared to DAPT <12 months.55 In another retrospective cohort study in Taiwan, involving mostly CCS patients (>70%), no significant difference was found between DAPT >6 months and DAPT <6 months in terms of risk of death, MI or bleeding among post-stenting patients on dialysis.56 Altogether, because of the markedly increased risk of bleeding among patients on dialysis relative to ischaemic risk, the expert panel voted in favour of the use of shortened DAPT in these patients, and to continue with SAPT among those transitioning to CCS therapy, with the aim of reducing bleeding risk while on the full course of antiplatelet therapy.51,57 Clopidogrel-based DAPT may also be recommended in those with severe CKD, including those on haemodialysis, due to the increased risk of bleeding in these patients.53
Some PCI strategies may render patients amenable to shortened DAPT. Indirect evidence suggests that a shortened DAPT may be used for a third-generation DES, with no additional risk of ischaemia. Among subjects in SMART-CHOICE who underwent PCI using Orsiro (Biotronik), a third-generation DES, there was no significant difference between shortened DAPT and standard DAPT in terms of target vessel failure.58 However, these indirect findings represent low-quality evidence to support the use of third-generation DES for patients with CKD in countries where these types of stents are available.
Table 2 summarises these consensus statements on the use of P2Y12 inhibitors in patients with CKD.
Elderly Patients
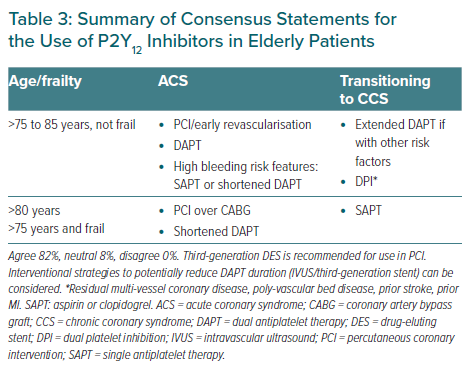
Statement 7. Elderly patients should be assessed for bleeding risk prior to P2Y12 inhibitor initiation.
Level of evidence: High.
Level of agreement: Agree 96%, neutral 0%, disagree 4%.
Statement 8. Elderly patients aged >75 years post-ACS/post-PCI with stent implantation should receive DAPT if there are no high bleeding risk features by ‘ABO’ criteria.
Level of evidence: Low.
Level of agreement: Agree 100%, neutral 0%, disagree 0%.
Statement 9. Elderly patients with unacceptably high bleeding risk may alternatively receive SAPT or shortened DAPT.
Level of evidence: Low.
Level of agreement: Agree 100%; neutral 0%, disagree 0%.
Statement 10. Caution for excessive bleeding should be exercised when giving DAPT to elderly patients aged >80 years. Shortened DAPT may be considered.
Level of evidence: Low.
Level of agreement: Agree 96%, neutral 4%, disagree 0%.
Statement 11. Ticagrelor has been shown to be effective and safe in elderly patients versus clopidogrel, but not prasugrel.
Level of evidence: Low.
Level of agreement: Agree 73%, neutral 19%, disagree 8%.
Statement 12. In patients aged ≥75 years, prasugrel is generally not recommended.
Level of evidence: Low.
Level of agreement: Agree 88%, neutral 8%, disagree 4%.
Statement 13. If aspirin would be included in SAPT or DAPT in elderly patients, low doses (75–100 mg) should be used.
Level of evidence: Low.
Level of agreement: Agree 100%, neutral 0%, disagree 0%.
Statement 14. Elderly patients on DAPT may receive a proton pump inhibitor (PPI).
Level of evidence: Low.
Level of agreement: Agree 92%, neutral 4%, disagree 4%.
Statement 15. Consider de-escalation strategies (shortened DAPT or appropriate dose reduction) for elderly patients, balancing ischaemic and bleeding risks.
Level of evidence: Low.
Level of agreement: Agree 92%, neutral 4%, disagree 4%.
Statement 16. In elderly CCS patients, frailty or age >80 years old are considered excessively high bleeding risk features and should receive SAPT.
Level of evidence: Low.
Level of agreement: Agree 88%; neutral 12%, disagree 0%.
A higher burden of co-morbidities and altered platelet function may add complexity to antiplatelet therapy in the elderly compared to younger populations.59,60
While cut-offs for age varied across the trials comparing shortened versus extended DAPT, age was generally not found to significantly influence the efficacy and safety of the treatment regimens.36,61–63 Nevertheless, despite the heterogeneous data from the trials, some evidence may be used to assist providers in tailoring antiplatelet therapy.
Among patients ≥65 years old in SMART-CHOICE and TWILIGHT, there was no significant difference in efficacy between standard DAPT and shortened DAPT followed by SAPT using P2Y12 inhibitor.34,36 In contrast, an exploratory, possibly underpowered, subgroup analysis of GLOBAL LEADERS showed shortened DAPT followed by ticagrelor monotherapy was associated with a significantly lower risk of ischaemia compared to standard DAPT among patients >75 years of age.61
Interpretation of safety data in the elderly is likewise complicated by conflicting results among the trials. In SMART-CHOICE, there was no significant difference in bleeding rates between standard DAPT and shortened DAPT followed by SAPT using P2Y12 inhibitor among participants ≥65 years old.36 However, in the same age group in TWILIGHT, bleeding risk was significantly lower with shortened DAPT followed by ticagrelor monotherapy compared to standard DAPT.34
GLOBAL LEADERS had a high enrolment of subjects with advanced age (7.3% were octogenarians, 1.5% were ≥85 years old).61 Although this study found no significant difference between treatment arms in terms of bleeding risk among subjects >75 years old, a further subgroup analysis of ACS patients showed a significantly lower bleeding risk with shortened DAPT followed by ticagrelor monotherapy.61 In contrast, the subgroup analysis of CCS patients revealed a higher bleeding risk from shortened DAPT followed by ticagrelor monotherapy.60 The investigators surmised this latter finding may have been biased because ‘stable’ CCS patients in the experimental arm took a more potent P2Y12 inhibitor (ticagrelor) than the control arm (clopidogrel).61
Regarding choice of antiplatelet agent in regimens for the elderly, high-quality evidence among Asian populations has been limited, which may explain the 19% neutral votes for Statement 11. In a Swedish observational study involving post-MI patients on DAPT, there was no significant difference between the ticagrelor group and the clopidogrel group in terms of combined ischaemic outcomes among patients ≥80 years old, but ticagrelor-treated patients were at higher risk of re-admission for bleeding.64
Lastly, the open-label, randomised controlled POPULAR AGE trial in the Netherlands found that in patients aged 70 years or older with non-ST-elevation ACS, clopidogrel was associated with lower bleeding rates compared with ticagrelor (p=0.02 for superiority) while having similar net clinical benefit outcomes (p=0.03 for non-inferiority).65 Given these findings, clopidogrel may be preferred over other P2Y12 inhibitors for elderly patients with high risk of bleeding.
Regarding the optimal duration of shortened DAPT, evidence in the elderly is similarly scant. In STOPDAPT-2, the duration of shortened DAPT was 1 month while in SMART-CHOICE and TWILIGHT, it was 3 months.34,36,38 Reflecting this variation, a range from 1 to 6 months of shortened DAPT constitutes the current practice in Asia.
The totality of the current evidence, therefore, shows that while potent P2Y12 inhibitors such as ticagrelor and prasugrel may be used in elderly patients, caution should be exercised in those with high bleeding risk (i.e. extremes of age and frailty). Caution has been advised regarding the use of prasugrel in patients ≥75 years old in previous recommendations.1,26 The panel acknowledges that certain Asian countries and regions use a lower-dose preparation of prasugrel (3.75 mg), which may reduce the risk of bleeding compared to the standard 10-mg dose. As supported by the GENERATIONS study, the use of prasugrel at lower doses (5 mg) may be considered when available, after careful evaluation of the impact of such dose reduction on the patient’s on-going ischaemic risk.66 In contrast, no dosing adjustments have been required for ticagrelor.1,26
Most trials implemented a low-dose aspirin regimen, consistent with the dose recommended in previous guidelines (75–100 mg daily). In previous guidance documents, PPIs have been recommended for the following groups: ACS patients on a P2Y12 inhibitor with high bleeding risk; CCS patients receiving aspirin or combination therapy at high risk of gastrointestinal bleeding; and patients on DAPT with risk factors for bleeding.1,24,26 Generally, in these past recommendations, advanced age composed a criterion for high bleeding risk warranting PPI initiation. A recent Danish retrospective cohort study showed that the use of PPI reduced the risk of gastrointestinal bleeding among patients on DAPT, including those belonging to ESC-defined high-risk groups.67 However, the overall baseline risk for bleeding while on DAPT was found to be low.67 Furthermore, these marginal benefits of PPI use should be weighed against the possibility that omeprazole and esomeprazole, being cytochrome P450 2C19 inhibitors, may reduce response to clopidogrel.68
Extreme old age or frailty constitute criteria for high bleeding risk used in considering the initiation of DAPT for CCS patients in ESC guidelines.1 In their 2020 recommendations for ACS patients, the ESC has described frailty as a clinical syndrome of decreased biological and physiological reserves that lead to impaired responses to stress (i.e. longer hospital stay, higher risk of death).68 A number of scales for frailty or physical performance (Short Physical Performance Battery, Rockwood Clinical Frailty Scale, Columbia Frailty Index and the Edmonton Frail Scale) may predict the occurrence of major bleeding while on DAPT, although the APSC does not endorse the use of any particular scoring system for frailty.69
High-quality evidence is lacking regarding the optimal antiplatelet therapy for patients with frailty or age >80 years old. Given this data gap, the expert panel referred to the 2019 APSC consensus statements on high-risk CCS, which suggest the use of SAPT in such patients where excessive bleeding risk has been identified.32 Therapy for frail patients must be individualised and must consider other factors such as life expectancy, quality of life, and patient preferences.70
Table 3 summarises the consensus statements for the use of P2Y12 inhibitors in elderly patients.
Diabetes
Statement 17. Antiplatelet therapy should be used for secondary prevention in patients with type 2 diabetes and established cardiovascular disease (with preference for ticagrelor for patients with low bleeding risks).
Level of evidence: High.
Level of agreement: Agree 96%, neutral 0%, disagree 4%.
Statement 18. For diabetes patients with an MI or undergoing PCI, an extended DAPT regimen can be considered.
Level of evidence: Moderate.
Level of agreement: Agree 88%, neutral 8%, disagree 4%.
Statement 19. For diabetes patients with complex PCI and high bleeding risk, ticagrelor monotherapy can be considered after 3 months of DAPT.
Level of evidence: Moderate.
Level of agreement: Agree 92%, neutral 8%, disagree 0%.
The propensity for both ischaemia and bleeding in diabetes may be explained by endothelial dysfunction and altered haemostatic and thrombotic mechanisms.71 The PEGASUS-TIMI 54 trial found that among patients with prior MI, extended DAPT using ticagrelor plus aspirin, significantly reduced the primary composite efficacy endpoint in all subgroups, including those with diabetes.63 This study also indicated that ticagrelor 60 mg had a numerically lower rate of bleeding while having similar efficacy to the 90 mg dose.
Furthermore, among diabetes patients in the THEMIS trial who had CCS but no prior stroke or MI, extended DAPT with aspirin plus ticagrelor significantly lowered the risk of ischaemic outcomes compared to SAPT with aspirin alone.62 However, because there was also significantly higher risk of bleeding associated with extended DAPT using aspirin plus ticagrelor, its use may need to be limited to patients with low bleeding risk.62 These findings show that there is a need for holistic assessment of ischaemic and bleeding risks among diabetes patients prior to initiation of DAPT.
In a subgroup analysis of the THEMIS trial, diabetes patients who had history of previous PCI experienced significantly reduced ischaemic outcomes with DAPT using aspirin plus ticagrelor compared to SAPT using aspirin alone.72 However, DAPT was also associated with a significantly increased risk of major bleeding in this subgroup.72 These findings suggest that in CCS populations with diabetes, SAPT remains the treatment of choice, but DAPT with ticagrelor can be considered for patients who have high ischaemic risk, such as patients with history of PCI, as long as they have low bleeding risk. In contrast, if they have high bleeding risk and had undergone complex PCI, instead of standard DAPT with ticagrelor, shortened DAPT for 3 months followed by ticagrelor monotherapy can be considered, as suggested by Asian evidence from TWIILIGHT.73
In European guidelines, diabetes confers a moderate risk for ischaemia and warrants consideration of adding a second antiplatelet agent to aspirin among CCS patients without high bleeding risk.1 On the other hand, American guidelines have listed diabetes as a risk factor for bleeding, ischaemia, and stent thrombosis and as criteria in the DAPT risk score used to determine DAPT duration.24
Multivessel Coronary Artery Disease
Statement 20. Extended DAPT (>12 months) can be considered for patients with multivessel CAD post-revascularisation (PCI or coronary artery bypass graft [CABG]).
Level of evidence: Low.
Level of agreement: Agree 73%, neutral 23%, disagree 4%.
Statement 21. Patients with multivessel CAD not amenable to revascularisation should receive antiplatelet therapy.
Level of evidence: Low.
Level of agreement: Agree 100%, neutral 0%, disagree 0%.
Apart from multivessel disease (i.e. ≥3 vessels), the definition of complex PCI in TWILIGHT involved a number of angiographic features such as total stent length >60 mm, bifurcation with two stents, use of any atherectomy device, left main artery as target vessel and surgical bypass graft or chronic total occlusion as target lesions.74 Among these criteria, total stent length >60 mm was reported as the most common in the trial.74 In European guidelines for CCS, multivessel CAD comprises a criterion for moderate risk of ischaemia and also for high risk when combined with one other risk factor, warranting consideration of the addition of a second antiplatelet agent to aspirin.1
Patients with multivessel CAD post-revascularisation may be considered for extended DAPT, based on the results of PEGASUS-TIMI 54, where almost 60% of patients had multivessel CAD and >80% had previous PCI. Importantly, this study also suggests that ticagrelor 60 mg had a numerically lower rate of bleeding while having similar efficacy to the 90 mg regimen.63
It should be noted that no direct evidence was found on the benefit of extended DAPT in post-CABG patients without a history of MI or stenting. Indirect evidence from CHARISMA, in which 26% of patients underwent PCI and 17% underwent CABG, suggests that clopidogrel plus aspirin for a median of 28 months was associated with a significantly lower rate of cardiovascular death, MI or stroke compared with aspirin alone (p=0.01).75 Among Asians with multivessel CAD, the current body of evidence on optimal DAPT duration is scarce. Nonetheless, given the high ischaemic risk in this subset of patients, the consensus panel agreed that extended DAPT may be considered post-revascularisation in patients with low bleeding risk. The Asian evidence in more specific subgroups, such as patients with a history of previous CABG, is even more limited, which may explain the number of neutral votes (23%) for Statement 20.
Patients with multivessel disease who are ineligible for revascularisation are similarly at high ischaemic risk. Although there has been no strong evidence available on the optimal treatment for this subgroup, the panel voted in favour of antiplatelet therapy to avert the high ischaemic risk.
Treatment Continuity After Bleeding During Antiplatelet Therapy
Statement 22. The decision to discontinue antiplatelet therapy in patients should be based on an assessment of the severity of bleeding and the proximity of the bleeding event to the index ischaemic event or PCI.
Level of evidence: High.
Level of agreement: Agree 100%, neutral 0%, disagree 0%. p>
Statement 23. As much as possible, patients with bleeding within 1 month of the index event should continue antiplatelet therapy once stabilised to minimise ischaemic risk.
Level of evidence: Low.
Level of agreement: Agree 88%, neutral 8%, disagree 4%.
Statement 24. Patients with bleeding who require a stepdown of antiplatelet therapy may consider switching to less potent antiplatelets, reduction in the antiplatelet dose or the use of single antiplatelet agents.
Level of evidence: Low.
Level of agreement: Agree 100%, neutral 0%, disagree 0%.
In an effort to contribute to knowledge on de-escalation of antiplatelet therapy, investigators of GLOBAL LEADERS studied the association between any reported bleeding or MI during therapy and mortality.76 They showed that bleeding or MI during therapy was significantly associated with a sustained higher risk for subsequent mortality from 30 days to beyond 1 year after the event. Furthermore, they found that switching to a less potent antiplatelet (i.e. from ticagrelor or prasugrel to clopidogrel or aspirin) or discontinuing any antiplatelet agent for >5 days at the time of Bleeding Academic Research Consortium (BARC) 3 bleeding significantly decreased the risk of subsequent bleeding or MI compared to continuation of therapy. However, there was no sufficient evidence that de-escalation or discontinuation of therapy during BARC 2 bleeding had the same benefit.76
There is very little evidence in Asia to provide definitive guidance on treatment discontinuation or de-escalation. Notwithstanding, among Asian patients, the bleeding risk post-ACS tends to be overestimated compared to the ischaemic risk.77 Therefore, antiplatelet therapy should be discontinued only after a thorough assessment of, firstly, the severity of the bleeding event and, secondly, its proximity to the index ischaemic event or PCI, a factor that correlates directly with the patient’s ischaemic risk (i.e. the closer to the index event, the higher the ischaemic risk). Accordingly, to minimise ischaemic risk, the panel voted in favour of continuing antiplatelet therapy if bleeding occurs within 1 month of the index event.
Limitations
While large-scale trials on P2Y12 inhibitor regimens have been conducted worldwide, there is still a need for further Asian-specific data for generalisation to Asian populations. Hence, some recommendations are based on expert opinion.
We did not perform meta-analyses, and these consensus statements are not exhaustive. Nonetheless, we have endeavoured to gather the best available evidence at the time of publication. Lastly, the consensus statements are not intended to replace clinical judgement.
Conclusion
Because of the increased risks for ischaemia and/or bleeding, special populations in CAD (i.e. advanced age, CKD, or diabetes) and particular settings (i.e. transitioning from ACS to CCS, presence of multivessel disease or bleeding during antiplatelet therapy) may require modification of standard therapy. The management of such cases may be different between Asian and Western populations. Some evidence from Asian trials supports the use of ticagrelor monotherapy during the transition to CCS among patients with high ischaemic and low bleeding risks. Although standard DAPT is generally recommended for CKD, elderly, and diabetes patients, there is some evidence to support the use of shortened DAPT or SAPT among those with high bleeding risk. Meanwhile, there is little evidence to provide definitive recommendations on antiplatelet therapy for multivessel CAD or de-escalation strategies after bleeding among Asian patients. In all situations, the risks for ischaemia must be weighed carefully with the risks for bleeding to individualise antiplatelet therapy accordingly.