Chest pain is one of the most frequent symptoms in patients seeking treatment in primary care and the emergency department (ED).1 It accounts for up to 6% of attendances to the ED and 20% of emergency admissions to hospital.2,3 The development and introduction of a standardised approach in the management of these patients through risk assessment scores has improved the time to diagnosis and reduced hospital admissions alongside mortality and morbidity.4
However, it is unclear as to whether a standardised approach can adequately triage chest pain events in cancer patients and survivors. These specific cohorts of cancer patients are particularly vulnerable, given the link between cancer and excess risk of heart disease. Moreover, cancer treatments, including chemotherapy, immunotherapy and radiation therapy, are associated with an increased risk in cardiovascular events that can persist for weeks or even years after the completion of treatment.5–7 In addition, nearly two-thirds of these patients are aged 65 years or older, with a higher prevalence of comorbidities than younger patients, making their evaluation a distinctive challenge.8 Last, there remains a concern that healthcare providers might falsely attribute symptoms of heart disease, such as fatigue and dyspnoea to cancer, therefore resulting in suboptimal management.9
It is paramount that healthcare providers are aware of the additional risks that cancer patients and survivors face, given that cardiovascular disease is now the leading cause of death in many cancer survivors, ahead of cancer recurrence. Unfortunately, patients with cancer have been excluded from most major cardiology trials and registries, and their cardiovascular management remains largely empirical and extrapolated from non-cancer cardiac patients.10 A multidisciplinary approach to the management of these complex patients, facilitated by the cardio-oncology team, will produce the best outcomes.11
This review will cover the most common causes of chest pain in patients with cancer, with an emphasis on its underlying mechanisms and how its management may differ to that of non-cancer patients with chest pain. It will also highlight the importance of prompt referral to the cardio-oncology team, who can aid in identifying cancer therapy-related cardiovascular side-effects and provide optimum multidisciplinary care for these patients.
Potential Causes of Chest Pain in Cancer Patients
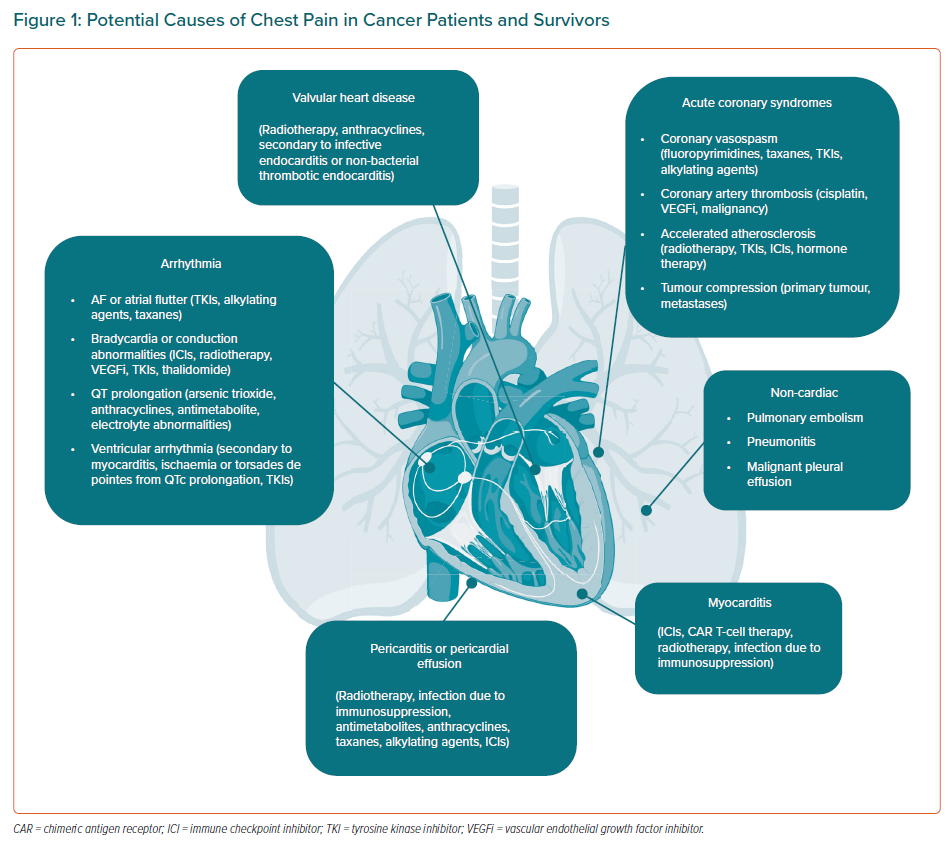
Cancer patients and survivors can present with chest pain due to multiple causes (Figure 1).
Acute Coronary Syndromes
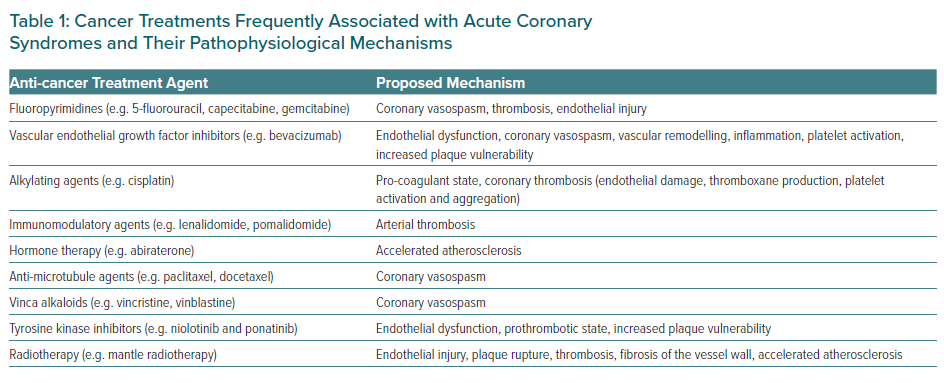
Coronary artery disease (CAD) and cancer often co-exist.12 A variety of anti-cancer therapies have a significant impact on the cardiovascular system, and can cause endothelial damage, vasospasm, platelet activation and/or aggregation, and attraction of elevated LDL cholesterol particles (Table 1). Cancer disease has been reported to be present in up to 17% of patients with acute coronary syndrome (ACS), and approximately 1 in 10 patients undergoing percutaneous coronary intervention (PCI) have either a current or historical diagnosis of cancer, with prostate, breast, colon and lung cancers being the four most common types of cancer encountered.13,14 Unfortunately, cancer patients requiring PCI are often at an increased risk of complications, adverse cardiac events and mortality, and the underlying causes are likely to be multifactorial.15
Different cancer subtypes are associated with different adverse outcomes. For example, colon cancer with metastases has the strongest independent association with major bleeding events, with almost a fivefold increase in risk.14 Patients with metastatic cancer, irrespective of cancer type, have a poorer prognosis after PCI and are at an increased risk of in-hospital mortality and PCI complications, including major bleeding events.14 A retrospective study by Borovac et al. showed that a lymphoma diagnosis is independently associated with an increased likelihood of adverse short-term clinical outcomes, higher rates of bleeding, vascular complications, and in-hospital mortality once adjustments for differences in baseline characteristics were applied in those undergoing PCI.15
Cancer patients with ACS tend to present more atypically compared with non-cancer patients. Less than one-third of cancer patients with ACS (30.3%) present with chest pain, 44% experience dyspnoea, and 23% present with hypotension.16 Therefore, it is important to have a higher clinical suspicion when screening cancer patients for ACS.
Initial investigations include a 12-lead ECG and serial serum troponin levels. An echocardiogram is helpful in determining regional wall motion abnormalities and left ventricular ejection fraction. Further investigations may include ischaemia testing or, when appropriate, invasive coronary angiography.
The management of these patients can often be challenging because they are often excluded from prospective studies and trials assessing the efficacy and safety of ACS treatment. As a result, their treatment is often not supported by a strong evidence base, and current guidelines for invasive and conservative treatment of ACS are not easily applied to all cancer patients. Potential concerns that may arise include the presence of anaemia, thrombocytopenia and coagulopathy in cancer patients.
Approximately 10% of cancer patients have platelet counts <100 × 109/l, which increases the risk of bleeding, the propensity for thrombus formation, and other adverse cardiac events.17 A prophylactic platelet transfusion should be considered at a threshold of 20,000/ml in patients with solid tumours, and for those with demonstrated necrotic tumours, due to the increased risk of bleeding at these sites.18 There is no minimum platelet count indicator for a diagnostic coronary angiogram.18 For platelet counts <30 × 109/l, revascularisation and dual antiplatelet therapy (DAPT) may be recommended after a preliminary multidisciplinary evaluation (interventional cardiology, cardio-oncology, oncology and haematology) and a risk–benefit analysis.18
For anaemic patients, red blood cell transfusion is generally recommended when haemoglobin is less than 7 g/dl, and consultation with haematology and oncology specialists is recommended for severely anaemic cancer patients undergoing cardiac catheterisation.18
Another important consideration is vascular access in cancer patients. For cancer patients who are suitable candidates for both radial and femoral access, the radial artery is generally preferred. In cancer patients on haemodialysis, those with abnormal Allen’s tests in both arms, multiple radial procedures or a-lines, bilateral mastectomy, or for whom a complex intervention is anticipated, femoral access may be the preferred approach.18
In general, when PCI is indicated, careful patient selection based on performance status, cancer prognosis, type of malignancy and anticipated cancer therapy can often determine the timing of the intervention, the access site, and the revascularisation approach. For example, in cancer patients with an expected survival of less than 1 year, percutaneous revascularisation may be considered for patients with acute ST-elevation MI and high-risk non-ST-elevation MI. In patients with stable angina, it would be reasonable to ensure that every effort made to optimise medical therapy before resorting to an invasive strategy.18 In the event that PCI is required in patients awaiting cancer surgery, balloon angioplasty without stenting or implantation with newer generation drug-eluting stents may be required in order to minimise and reduce the duration of DAPT, given that any interruption in DAPT may lead to in-stent thrombosis, especially in the types of cancer with an increased propensity for thrombosis.
Due to the above considerations necessary in cancer patients presenting with ACS, the need for early referral to the cardio-oncology team to facilitate multidisciplinary decision-making on a case-by-case basis is imperative.
Mechanisms of ACS in Cancer Patients
The mechanisms of CAD and ACS can differ significantly in cancer patients compared with the general population.19
These findings underscore the importance of both primary and secondary prevention in this population. Clinical evaluation and, when necessary, testing for detection of myocardial ischemia is key to identifying patients with latent pre-existing CAD. This may have implications for the selection of cancer treatment.20
Coronary Artery Vasospasm
Fluoropyrimidines, which include 5-fluorouracil (5-FU) and capecitabine, form the cornerstone of several different chemotherapy regimens and are known to cause MI via coronary artery vasospasm in cancer patients, with an incidence of 2–34% for 5-FU and 3–9% for capecitabine.21–24 These agents alter the tone of vascular smooth muscle cells. Pre-existing CAD remains a risk factor for fluoropyrimidine-related vasospastic angina, which most probably reflects the observation that vasospasm tends to occur at sites of thrombus and plaque formation; however, its exact underlying mechanisms have yet to be fully understood.25,26
Patients on 5-FU or capecitabine presenting with chest pain should have their fluoropyrimidine infusion ceased immediately, followed by treatment with medical anti-anginal therapy aimed at symptomatic relief, such as calcium channel blockers and/or nitrates, which have been shown to abolish symptoms in up to 69% of affected patients.23 Once a patient has been diagnosed with fluoropyrimidine-related cardiotoxicity, rechallenge is not advised in most patients, given that rates of recurrence of cardiotoxicity as high as 90% have been reported, along with a mortality rate of up to 13%.23 However, careful consideration of the risks against the potential benefits of re-treatment is advised for each individual.27
Other chemotherapeutic agents that can cause coronary artery vasospasm include vascular endothelial growth factor inhibitors (VEGFi), with an incidence of 1–15%.28 Paclitaxel and docetaxel can induce severe coronary vasospasm, which has also been reported to cause ACS.
Hypomagnesaemia, which frequently accompanies cisplatin therapy, may provoke vasospasm of the coronary arteries.29,30
Chemotherapy may be continued when results from all non-invasive and/or invasive tests are normal.21,22 Any potentially modifiable risk factors and diseases should be optimised prior to rechallenge with vasotoxic therapy.
Coronary Artery Thrombosis
Cancer is recognised as a prothrombotic venous and arterial state and the incidence of arterial thrombosis specifically is higher in cancer patients, in whom spontaneous coronary thrombosis without underlying atherosclerosis has been observed.31 This may occur through circulating microparticles, secretion of procoagulant factors, and alterations in platelet activity and endothelial function.32 Patients across all age groups with myeloproliferative neoplasms are at significant risk compared with matched controls, with the highest rates at and shortly after diagnosis.33 Advanced cancer stage is also associated with an increased risk of arterial thromboembolism; in patients who do develop arterial thromboembolism this carries a poor prognosis, with a threefold increased HR for death compared with non-cancer patients.31
Chemotherapeutic agents may also increase the risk of arterial thrombosis. Cisplatin is associated with a 6–10% risk of venous or arterial thrombosis, while VEGFi are associated with a 3.8% risk of arterial thrombosis.34,35
Treatment options include acute percutaneous recanalisation of the blocked artery when indicated. The optimal anti-thrombotic strategy to treat acute arterial thromboembolism in patients with cancer is, however, unclear. This cohort of patients sometimes receive empirical long-term anticoagulation because of concerns regarding cancer-mediated hypercoagulability, however, this decision is best made via close collaboration with the patient’s oncologist and/or haematologist and cardiologist.
Atherosclerosis/Accelerated Atherosclerosis
CAD is more prevalent in cancer patients due to their shared risk factors (e.g. obesity, smoking, age, sedentary lifestyle).36,37 Cancer therapies may also lead to an increased rate of coronary atherosclerosis and therefore the incidence of cardiovascular events has been increasing in younger patients over the years. Radiation causes vascular endothelial damage, which in turn promotes inflammation and accelerates atherosclerosis. Coronary ostia and proximal segments are typically involved, and the most exposed coronary arteries are the left anterior descending artery during left breast irradiation and the left main stem, circumflex, and right coronary arteries during treatment for mediastinal Hodgkin’s lymphoma.38
For cancer survivors the incidence of fatal MI in patients treated with mediastinal radiation therapy is 1.5–threefold greater than in those who had not received radiation therapy, with the risk of damage heavily dependent on the dose and radiation field.39,40 In this cohort of patients, the indications for screening with non-invasive cardiac imaging remain unclear. However, aggressive cardiac risk factor modification and follow-up in late-effects clinics are essential.
Chemotherapeutic agents may also cause accelerated atherosclerosis. Such agents include VEGFi, tyrosine kinase inhibitors (TKIs), immune checkpoint inhibitors (ICIs) and hormone therapies such as abiraterone. Common cardiovascular events during treatment with these agents include rapidly progressive peripheral artery occlusive disease and acute myocardial ischaemic events.
Acute Coronary Syndrome via Tumour Compression
Patients with cancer can also develop signs and symptoms of myocardial ischaemia because of coronary artery compression by various primary and secondary cardiac tumours. Primary angiosarcoma of the coronary arteries leading to ACS is extremely rare.41 ACS secondary to tumour compression usually signifies advanced disease and management should be in close consultation with the oncology team.42
Myocarditis
Contemporary anticancer immunotherapy has changed the landscape of treatment for patients with a variety of malignancies who historically had a poor prognosis. However, any therapy that modulates the immune system has the potential to be associated with myocarditis. Most recently, this clinical entity has been most observed in the setting of ICIs and chimeric antigen receptor (CAR) T-cell therapy.43–45
The incidence of ICI-associated myocarditis is unclear given the lack of routine cardiac monitoring in most immunotherapy trials; however, it has been reported to range from 0.06% to 1% of patients prescribed an ICI, with its prevalence reported to be higher with combination immune therapies. Although the risk factors for ICI-associated myocarditis are not well characterised, diabetes and obesity have been independently associated with higher occurrence.46 ICI-associated myocarditis occurs early at a median time of 1–2 months, with most of the cases occurring within 3 months after starting ICI therapy.43,47
Patients typically present with a wide range of symptoms including chest pain, shortness of breath, non-specific symptoms, such as fatigue and myalgia, and in some instances, sudden cardiac death.48,49 Clinicians should be vigilant for immune-mediated myocarditis, particularly due to its early onset, non-specific symptomatology and fulminant progression. Initial investigations include a 12-lead ECG and serial troponin levels. Echocardiographic findings may vary from a normal examination to reduced thickening, reduced global longitudinal strain, regional and global wall motion abnormalities and/or diastolic dysfunction.46,50 Cardiac MRI also offers helpful information regarding the presence of prior MI scar, diffuse fibrosis and interstitial oedema.51
With regards to treatment, although data from rigorous studies of treatment for immune-related adverse events are unavailable to date, consensus guidelines recommend high-dose steroids with progressive tapering, dependent on patient symptoms and cardiac troponin levels.52 In cases in which an improvement is not seen, other immunosuppressant agents such as infliximab, rituximab and mycophenolate mofetil can be considered.52 Rechallenge may be considered (often with a single agent) in the context of an individualised approach after a multidisciplinary discussion.
Another possible anti-cancer treatment that may cause myocarditis is CAR T-cell therapy. CAR T-cell immunotherapy is associated with potentially life-threatening cytokine release syndrome, which in turn may lead to myocardial injury, arrhythmia, cardiomyopathy and circulatory collapse.45,53,54 It usually develops early on after CAR T-cell infusion (median time, 2.2 days) with the highest risk in the first 2 weeks after infusion.55 Fortunately, current data suggest that CAR T-cell-related cardiovascular complications are acute and transient, with the incidence of persistent left ventricular systolic dysfunction at 6 months being very low, and with no late cardiovascular effects observed at 1-year follow up.56,57
Supportive treatment, as well as tocilizumab, an anti-interleukin-6 receptor antibody, is the cornerstone of treatment. Recent findings suggest that pre-existing cardiovascular risk factors and disease may increase the risk of such cardiotoxicity, and prompt recognition, as well as treatment, may favourably alter the outcomes.58
In essence, a multidisciplinary approach is crucial for the management of patients on novel immunotherapies, and cardio-oncologists play a fundamental role in the comprehensive care of these patients.
Another less common cause of myocarditis in cancer patients is radiation myocarditis secondary to high-dose radiation treatment. This can eventually result in myocardial fibrosis with subsequent restrictive cardiomyopathy. Management of radiation-caused cardiomyopathy is similar to the treatment of other types of cardiomyopathy and is typically symptomatic treatment.59
Pericardial Disease
Malignant involvement of the pericardium is detected in 1–20% of autopsy studies of cancer patients.60 The spectrum of the acute clinical presentation can include acute pericarditis, pericardial effusion with or without cardiac tamponade, or pericardial constriction. Pericardial disease in cancer patients can be as a result of their cancer treatment, such as chemotherapy or radiotherapy. Determining the aetiology and providing effective treatment can often be challenging.
Pericarditis
Pericarditis is the commonest form of pericardial disease and is responsible for around 5% of chest pain presentations to EDs.61,62 Mortality rates can reach 1%.63 In an unselected cohort of pericarditis patients, approximately 5% of cases were attributable to underlying cancer.62
The underlying mechanisms responsible for acute pericarditis in cancer patients include direct metastasis to the heart, pericardial haemorrhage, infections due to immunosuppression, and cancer therapies such as fludarabine, cytarabine, doxorubicin, docetaxel and cyclophosphamide, as well as radiation therapy. Acute pericarditis can occur during radiotherapy itself or within weeks after radiotherapy.64–66 ICIs may also cause acute pericarditis, and it can occur with coexisting myocarditis.67
The treatment of acute pericarditis is usually as per recommended guidelines in the general population. However, it should be noted that many cancer patients may have a predisposition to bleeding due to abnormal blood counts or coagulation abnormalities secondary to their disease or treatment. It can thus be challenging to introduce routine therapy such as non-steroidal anti-inflammatory agents in this context. As a result, there is often a greater and earlier use of other agents, for example colchicine and steroids, although this may not alter outcomes.63 In the case of radiotherapy-induced acute pericarditis, treatment of the primary malignancy should not be withheld because of this.68
Pericardial Effusion and Cardiac Tamponade
Cancer has been noted to be the most common cause of pericardial effusion in the Western world.69,70 In cancer patients, pericardial effusion can be caused by cancer invasion or it can occur secondary to anti-cancer treatment including radiation therapy, or secondary to infection due to underlying immunosuppression. The presence of a pericardial effusion in cancer patients carries significant morbidity and mortality.62,71,72
Patients with pericardial effusions or tamponade can present with symptoms such as syncope, chest pain or palpitations. The symptoms could also be subtle, such as dyspnoea, non-specific chest discomfort and simple fatigue.
The mainstay of treatment is to allow sufficient drainage of the pericardial fluid to relieve the symptoms and prevent recurrence. Pericardiocentesis is an easier and less invasive procedure than pericardial window surgery, enabling prompt treatment at the time of diagnosis. However, pericardiocentesis leads to a recurrence rate of up to 20% at 30 days, which is higher than the rate of recurrence after surgical drainage (1–10%). Rarely, malignant pericardial effusions are managed with intrapericardial injection of chemotherapeutic agents.73
Pericardial Constriction
In cancer patients, constrictive pericarditis can be caused by radiation exposure or chemotherapy, or it can occur as a sequela of previous episodes of pericarditis in which scarring with loss of pericardial elasticity has occurred over time. Cancer patients may present with non-specific chest pains although more commonly may report fatigue and exertional dyspnoea. The definitive treatment for chronic constrictive pericarditis remains pericardiectomy performed in experienced cardiac centres.
Arrhythmias
Rhythm abnormalities can occur in cancer patients due to anti-cancer treatment, potential drug–drug interactions during the course of treatment and metabolic and electrolyte derangements. The real incidence of cancer therapy-induced arrhythmias is likely to be underestimated because routine cardiac monitoring is often not performed.
AF is the most common sustained arrhythmia and is increasing in both prevalence and incidence.74 Currently, the prevalence of AF in cancer patients ranges between 2% and 15%, with higher rates reported for certain classes of antineoplastic drugs such as TKIs.75 In addition, cancer and AF share common risk factors such as age, smoking, alcohol use and obesity.76
Patients with AF are at an increased risk of cerebrovascular events, and anticoagulation may be necessary to reduce such risks. Recent comparative effectiveness data for patients with AF and cancer consistently have shown that direct oral anticoagulants are associated with lower or similar risks of bleeding and stroke compared with warfarin.77 Ultimately, multidisciplinary care that accounts for individualised risk factors, patient preference and periodic clinical reassessment is warranted to identify the optimal anticoagulation regimen.77
Other arrhythmias include conduction disease, QT prolongation and ventricular tachycardias.
Patients may present with chest pain although more commonly may report palpitations, pre-syncopal symptoms or symptoms related to heart failure. A proportion of patients would be asymptomatic, with the diagnosis being made only on routine monitoring.
The role of the cardio-oncology team in the prevention and management of arrhythmias in cancer patients is multifold, and includes baseline assessment upon cancer diagnosis, monitoring and management during active cancer, and long-term surveillance in cancer survivors.
Valvular Disease
Valvular Disease Secondary to Radiation Therapy/Chemotherapy
Radiation exposure is a risk factor in the development of clinically significant valvular heart disease. In a post-mortem series the incidence was high, with 81% of patients who had received radiotherapy in the past showing evidence of mild valvular damage.78 There is a latent interval of 10–20 years between radiation exposure and the development of clinically significant heart valve disease.
Patients may present with chest pain (more commonly seen with aortic stenosis), however, they are more likely to report symptoms of heart failure such as worsening shortness of breath on exertion and peripheral oedema.
Radiation-associated valvular disease is a complex disease that requires an integrated and multidisciplinary approach. With regard to patients suitable for percutaneous or surgical intervention, individualised timing and technique are critical, and therefore these patients should be managed at high-volume centres with experience in managing radiation-associated valvular disease.79
Non-cardiac Chest Pain
It is important to consider other causes of chest pain that may be non-cardiac in origin. These include pulmonary embolism given that it is more commonly seen in patients with cancer who have a sevenfold increased risk for venous thromboembolism, with an overall absolute risk of 7% in the first year of a cancer diagnosis and up to 20% depending on the type of cancer and treatments used.80–82 Other respiratory causes include malignant pleural effusions and pneumonitis, which can occur secondary to anti-cancer therapies including radiation therapy. Gastrointestinal pathology is another frequent cause of non-cardiac chest pain in patients with upper gastrointestinal cancers. This can be secondary to dyspepsia, obstruction from local tumours or strictures from previous radiotherapy treatment.
Conclusion
As life expectancy continues to rise, one of the largest challenges ahead will include the management of a significantly larger proportion of patients with cancer and concomitant cardiac disease. Increased awareness of the potential cardiovascular toxicity profile associated with the various cancer therapies enables appropriate cardiac surveillance that will encourage early detection and institution of treatment. Prompt referral to the cardio-oncology team is essential to ensure that patients’ cardiovascular needs are addressed throughout their entire cancer journey: before (risk assessment), during (detection of CV toxicity), and after (survivorship) cancer treatment. Equally, given that cardiac events associated with newer agents have a highly variable incidence and onset, healthcare professionals are encouraged to continuously educate patients about the potential cardiotoxicity associated with chemotherapy, and the need for ongoing monitoring during chemotherapy as well as long-term follow-up to assess for late cardiovascular complications.