Chronic kidney disease (CKD) and cardiovascular disease (CVD) are co-prevalent conditions with distinct epidemiological characteristics. Nearly 15% of the adult population in the US is affected by CKD, whereas end-stage kidney disease (ESKD) affects a smaller population; in the year 2018, ~554,038 patients were estimated to receive dialysis, and 229,887 patients had a functional kidney transplant in the US.1 The burden of CVD is nearly twofold higher among individuals with CKD versus without CKD (66% versus 32%).2 Among ESKD patients on dialysis, prevalent CVD is estimated to be a staggering 77%, with that of coronary artery disease (CAD) about 44%.1 The presence of CKD is associated with worse CVD outcomes. In an ambulatory population of 1.1 million adults, worsening kidney function was demonstrated to have a graded and independent association with all-cause mortality and CVD events.3 Similarly, in a collaborative meta-analysis, CKD was an independent predictor of all-cause and CVD mortality.4 The presence of CKD adversely impacts survival following any CVD event. Estimated 2-year adjusted survival following acute MI is ~87% without CKD, ~75% in CKD stages 4–5, ~53% in dialysis and ~77% in kidney transplant recipients.1
Clinical Presentation and Outcomes
Patients with CKD have a greater likelihood of acute rather than stable presentations with CAD.5 Importantly, patients with advanced CKD/ESKD are less likely to experience chest pain or have diagnostic electrocardiographic findings.6–8 It has also been demonstrated that in-hospital mortality with acute MI is exponentially higher in the presence of CKD compared with the absence of CKD.6,8–10 Multiple potential aetiopathogenic factors have been postulated to be contributory. Atypical clinical presentations in AMI may be a potentially contributory factor, and it has also been consistently demonstrated that patients with CKD receive fewer evidence-based therapies, including reperfusion/revascularisation therapies.9,10 Although it is problematic to derive any causal conclusions from associations from observational data, there has been concern raised about potential therapeutic nihilism or ‘renalism’ in this population. Based on data from contemporary studies, reassuringly, mortality from AMI has been declining in CKD/ESKD.11,12
Pathophysiology: What is Unique?
Understanding the unique aspects of CAD in CKD is important for identifying specific targets in this high-risk population. Autopsy studies in patients with advanced CKD and on dialysis identified more calcified and extensive atherosclerotic lesions. Invasive coronary angiography (ICA) data demonstrate fourfold higher relative risk of having multivessel CAD among patients with moderate-to-severe CKD, as compared with those with mild/no renal insufficiency, after controlling for the effect of diabetes.13 Patients with CKD, who underwent ICA prior to and during AMI, had a greater number of coronary plaques, specifically with >50% stenosis.14 There were also many similarities between characteristics of coronary plaques progressing to AMI in patients with and without CKD, demonstrating that degree of stenosis is not the only defining characteristic of coronary atherosclerosis in CKD. This suggests that although traditional pathophysiology is in play, it does not fully explain the heightened risk for CVD events, including AMI, experienced by patients with advanced CKD.
It is likely that there is a reciprocal relationship between coronary atherosclerotic process and renal function: the presence of atherosclerotic CAD has been demonstrated to be associated with worsening kidney function and vice versa.15,16 Much of the contribution of CKD to atherosclerosis progression lies in the heightened inflammatory milieu associated with decreasing renal function that may enhance the atherosclerotic process independent of traditional risk factors.17 This inflammatory phenotype of the endothelium was similarly observed in vascular tissue from children with CKD requiring dialysis therapy.18 Sanchis et al. proposed the terminology ‘inflammaging’ to conceptualise the process of premature vascular senescence due to dysregulated metabolism promoting oxidative DNA damage and pro-inflammatory substrate.18
Another component of coronary atherosclerosis that has become a useful diagnostic tool is calcification. Coronary artery calcification (CAC) in the general population correlates very well with sites of atherosclerotic plaque. Patients with CKD demonstrate greater degrees of plaque calcification, as well as premature and progressive vascular calcification. A recent meta-analysis of 47 studies in patients with various stages of CKD and kidney transplantation identified a pooled prevalence of CAC across CKD stages of ~60%, with a nearly two- to fourfold associated increase in all-cause and CV mortality.19 The calcification process is promoted in CKD for a number of reasons, including enhanced exposure to the substrates of calcium and phosphorus in the setting of metabolic bone disorders, as well as an imbalance favouring calcification promoters (e.g. receptor activator of nuclear factor-κB, and receptor activator of nuclear factor-κB ligand) over inhibitors (e.g. klotho, osteoprotegerin and fetuin A).20,21 CKD is characterised by extensive vascular calcification involving not just the tunica intima, but also extending into the tunica media.22 In addition, vitamin K metabolism is deranged in CKD, leading to further reductions in inhibitors of vascular calcification (e.g. matrix GLA protein).23 This is compounded when vitamin K antagonists (e.g. warfarin) are used in advanced CKD patients. Use of vitamin K antagonists have been associated with greater valvular and vascular calcifications in CKD patients.24,25
The high incidence of non-ST-elevation MI and the prevalence of heart failure with preserved ejection fraction/diastolic dysfunction in CKD suggests a unique process distinct from the typical coronary atherosclerotic plaque rupture with subsequent myocardial wall injury and progressive systolic dysfunction seen in the general population. Aside from the larger coronary vessels, the microcirculation perfusing the myocardium is being examined as an important contributor of such myocardial dysfunction. Microcirculation dysfunction – evaluated as poor coronary flow reserve and overall capillary density – is impaired in CKD.26,27 Contributing factors include left ventricular hypertrophy associated with long-standing hypertension, which is common in CKD.
Finally, the complex interplay of pathophysiological dysregulation between the heart and the kidneys is increasingly well-recognised and referred to as cardiorenal syndromes. To encompass the wider spectrum of bidirectional dysregulation, two major phenotypic categories have been proposed: cardiorenal and renocardiac syndromes.28 In composite, five distinct phenotypic subtypes have been described in the literature contingent upon acuity of presentation, as well as the sequence of organ involvement.28
Non-invasive Evaluation for Stable Coronary Artery Disease
In light of the high burden of CVD and CV mortality among individuals with CKD/ESKD, what is the ability of non-invasive testing to augment prognostic data?29,30 Traditionally, dobutamine stress echocardiography and single-photon emission CT myocardial perfusion imaging were employed. In general, these studies demonstrated prognostic utility in risk prediction of adverse CV outcomes and mortality in CKD/ESKD.31–36 However, their diagnostic accuracy (sensitivity/specificity) to predict obstructive CAD is suboptimal in this population relative to the general population.37 This was most recently demonstrated by the low prevalence of obstructive CAD in the invasive arm in the ISCHEMIA-CKD trial, despite the inclusion criteria of moderate/severe ischaemia on cardiac stress testing.38 Several factors can be postulated to adversely impact test accuracy due to various factors, including higher prevalence of obstructive CAD, significant left ventricular hypertrophy, endothelial dysfunction and reduced coronary flow reserve.
Coronary CT angiography has been shown to be a strong predictor of future events in the population of subjects without renal disease, but has traditionally been used with trepidation in CKD/ESKD patients, for concern that the high burden of CAC may confound assessment, as well as the risk of contrast nephropathy.39–41 In a study evaluating individuals undergoing coronary CT angiography (n=1,541), CKD remained a strong, independent predictor of all-cause mortality, CV mortality and MI; however, increased risk of CV mortality in CKD patients was driven by non-coronary CV deaths.42 These findings suggest that CV mortality in individuals with advanced CKD is not driven solely by obstructive CAD, but rather non-atherosclerotic CV events (e.g. heart failure and arrhythmias). This observation has important implications pertaining to the predictive capabilities of non-invasive stress testing (which evaluate for obstructive CAD) in predicting CV events in CKD – which cannot be completely predicted by non-invasive (or invasive) testing. CAC may also be considered to guide primary prevention among those with asymptomatic CKD without known CVD.43 Finally, assessment of coronary flow reserve by PET reflects not just epicardial stenosis, but also diffuse atherosclerosis and microvascular dysfunction, and has been shown to have prognostic utility in this population.44,45
Special Considerations: Kidney Transplant Evaluation
The preponderance of data regarding non-invasive evaluation in CKD/ESKD patients is based in the pre-renal transplant population, where the goal of non-invasive evaluation is to decrease the risk of cardiac morbidity/mortality post-transplant in mostly asymptomatic patients. There have been a multitude of small studies comparing different stress testing modalities for obstructive CAD prior to renal transplant. In a systematic review and meta-analysis, non-invasive imaging and ICA had similar predictive accuracy to identify future adverse CV events in advanced CKD; and a significant proportion of transplant candidates experienced adverse events despite prior negative stress tests.46 Again, these findings highlight the importance of contribution of non-coronary CV events as a contributor towards all-cause and CV mortality in this population. As in the general CKD population, coronary CT angiography has also been evaluated and found to have value in pre-transplant evaluation.47,48 Interestingly, post hoc analysis of the ISCHEMIA-CKD trial has brought into question the role of routine revascularisation in kidney transplant candidates, which may, in turn, impact patterns of upstream non-invasive testing.49 The ongoing and eagerly anticipated CARSK trial will address whether eliminating screening tests for occult CAD in those wait-listed for kidney transplantation is non-inferior to regular screening for the prevention of major CV events.50 However, by virtue of its design, this trial will only address management of wait-listed individuals and not the upstream question of whether/in whom/which initial non-invasive CV testing should be performed in this population.
Medical Management of Coronary Artery Disease
Data to guide medical management of CAD in advanced CKD/ESKD patients is limited, as this group has been excluded from most clinical trials.51 This is particularly the case when it comes to anti-thrombotic agents: although at increased risk for thrombotic events, they are paradoxically at increased risk for bleeding complications as well, and, therefore, particularly prone to exclusion. Based on the pathophysiology at play, antiplatelet and anticoagulant drugs may have different effects and efficacies in CKD compared with those without CKD. In a meta-analysis of antiplatelet agents in CKD, among individuals with acute coronary syndrome (ACS; n=9,969), antiplatelet agents compared with standard care had no significant effect on all-cause/CV mortality/MI, but were associated with serious bleeding.52 In a stable group of patients (n=11,701), antiplatelet agents decreased the risk of MI while increasing the risk of minor bleeding, with uncertain effects on major bleeding and mortality.
When considering dual-antiplatelet therapy (DAPT) after percutaneous coronary intervention (PCI), a meta-analysis of five trials involving 1,902 participants with moderate CKD showed that a short duration of DAPT (≤6 months) and an extended duration of DAPT (≥30 months) have a similar incidence of the combined endpoint of all-cause mortality, MI, stroke and stent thrombosis when compared with a 12-month DAPT duration.53 The risk of major bleeding was also similar. The recent ESC guidelines for the management of ACS remind us that there continues to be insufficient evidence to assess the safety and efficacy of P2Y12 receptor inhibitors in stage 5 CKD/ESKD, although DAPT is routinely prescribed in these patients following PCI.54 There is a growing body of data regarding the use of direct oral anticoagulants for secondary prevention of CV disease. The benefit of rivaroxaban and aspirin was superior to aspirin alone in CKD; however, the risk of major bleeding was higher.55 As always, the dual risks of bleeding and thrombosis make it precarious navigating the narrow route of safety between Scylla and Charybdis with direct oral anticoagulants.56
Statins form the mainstay of management of CAD in the general population, but their use in the CKD population is more controversial. The SHARP trial enrolled 9,270 patients with CKD without known CAD, and demonstrated reduction in major adverse cardiac events with a combination of simvastatin 20 mg and ezetimibe 10 mg daily.57 In the ESKD population on dialysis, the results of the 4D and AURORA trials highlighted no benefit in reduction of major adverse cardiac events with use of statins.58,59 A subsequent meta-analysis by the Cholesterol Treatment Trialist’s group demonstrated that despite a waning effect of therapies that lower LDL cholesterol (statins, ezetimibe) on clinical outcomes in ESKD compared with earlier stages of CKD, the overall treatment interaction by CKD stage was not significant.60 This observation suggests their potential beneficial role in all stages of CKD. The treatment of hypertension is beneficial in reducing CV events in CKD patients; intensive blood pressure control compared with standard blood pressure control in CKD patients without diabetes showed a strong trend (albeit non-statistically significant) towards the reduction of major adverse cardiac events.61 As in this instance, unfortunately, most evidence in CKD patients is based on subgroup analysis and is often underpowered or of low statistical quality.62 The optimal target blood pressure and most efficacious antihypertensive agent in decreasing CV risk in persons with CKD has not yet been established, especially in ESKD.63
Diabetes is a strong risk factor for CV disease, but there is insufficient data to suggest that management of diabetes can decrease the risk of CV disease in persons with CKD.64 Newer antidiabetes drugs that have CV benefits in the overall population are now being investigated in CKD patients. The DAPA-CKD trial enrolled patients with an estimated glomerular filtration rate (eGFR) of 25–75 ml/min/1.73 m2, and showed that compared with placebo, dapagliflozin decreased the risk of the primary outcome of decline in eGFR, ESKD, renal death or CV death, as well as the risk of the pre-specified secondary endpoint of death from CV cause and hospitalisation for heart failure.65 A pre-specified analysis of the trial showed that the decrease in all-cause mortality in the trial was driven by a lower incidence of non-CV death from infection/malignancy rather than a lower risk of CV mortality; the study was underpowered to detect a difference in the risk of non-fatal MI.66 As a group, the sodium–glucose cotransporter 2 (SGLT2) inhibitors have demonstrated marked reduction in CV events extending to eGFR values as low as 30 ml/min/1.73 m2.67 These agents are a promising new addition to the armamentarium for reducing overall CV risk (albeit not CAD alone) in CKD.
Impact of AF
AF is prevalent in CKD patients, and a further complicating factor in the selection of appropriate antiplatelet and anticoagulant agents. CKD itself is a risk factor both for AF and stroke, and patients with CKD are paradoxically at an increased risk for bleeding with or without anticoagulation.68 In general, direct oral anticoagulants are non-inferior to warfarin, with a lower risk of bleeding in CKD. There are insufficient prospective data to guide anticoagulation selection in patients with CKD, although apixaban is increasingly used based on pharmacokinetic data/retrospective analyses, especially in ESKD.68,69 There are no randomised data to guide the selection of combination antiplatelet and anticoagulant therapies following PCI in CKD patients with AF, but given the increased risk of bleeding associated with advanced CKD, ‘triple therapy’ in general should be avoided. A reasonable proposed approach is to employ clopidogrel and oral anticoagulation (without aspirin) for 12 months, followed by oral anticoagulation alone.70 However, careful individualisation by the clinician is necessary based on many different variables.
Potential Role of Renal Therapeutics
Complications of reduced eGFR that deserve attention for minimising the risk of CAD events include CKD-mineral bone disease (MBD), anaemia and reduced GFR itself. CKD-MBD is associated with accelerated calcification of the arterial system, including the coronary arteries.40,71 However, the effects of management of CKD-MBD on CAD events have been mixed. Most randomised trials of management of various components of CKD-MBD (parathyroid hormone reduction or phosphorus reduction) have not demonstrated a reduction in mortality or ACS. In the EVOLVE trial, treatment with the calcimimetic, cinacalcet, did not reduce the incidence of death, MI or unstable angina.72 In secondary analysis of treatment effect on subtypes of causes of death (overall CV and sudden death), there was evidence of benefit from treatment with cinacalcet.73 Despite the post hoc nature of the analysis, the benefits of reduction of parathyroid hormone appear to be towards non-atherosclerotic events.
Reduction in phosphate intake is an important component of CKD-MBD management. This is primarily accomplished by the ingestion of phosphate binders with meals (as dietary phosphate restriction is notoriously difficult to achieve). These binders have primarily been calcium-based or non-calcium-based (aluminium-based binders are no longer used). There have been concerns of increased non-skeletal calcification with prolonged use of calcium-based binders, so several studies have examined the relative benefit of non-calcium binders to calcium-based binders.74,75 Both studies revealed a marginal benefit with non-calcium binders (specifically sevelamer) for all-cause mortality, but not for specific CV events. In contrast, specific serum phosphorus targets have recently been demonstrated to reduce CAC in patients on haemodialysis. However, longer-term studies are required to determine if this would result in clinical endpoint improvement.
Despite limited evidence for control of metabolic parameters of renal disease and modification in coronary disease risk, there are some recent findings that deserve mention. More recently, Isaka et al. examined optimal phosphate level control in patients on haemodialysis in a 2 × 2 factorial design prospective randomised trial.75 Targeting a strict phosphate control of <4.5 mg/dl over the course of 12 months was associated with a slower progression of CAC, as compared with liberal control (5–6 mg/dl), but the type of phosphate binder used was not significantly associated with change in CAC. Finally, a recently developed chelating agent (SN472) was demonstrated to slow the progression of CAC in haemodialysis patients with elevated CAC, although this is still in early stages of a clinical trial.77
Anaemia is another common complication of CKD. The coexistence of anaemia and CKD has been associated with increased risk of fatal and non-fatal MI, as well as worse clinical outcomes following PCI.78–80 No randomised controlled trial has demonstrated a benefit of correcting anaemia on clinical outcomes (all-cause mortality or CV events) in non-dialysis and dialysis-requiring CKD.81–83 The benefits of the novel hypoxia-inducible factor inhibitors for the treatment of anaemia of CKD on cardiovascular outcomes is yet to be determined. Ultimately, correction of uraemia is required to reverse the overwhelming effects on the CV system. Currently, this is best accomplished via kidney transplantation. Studies have demonstrated the consistent reduction in mortality and CV events following kidney transplantation, as opposed to staying on dialysis.84
Coronary Revascularisation
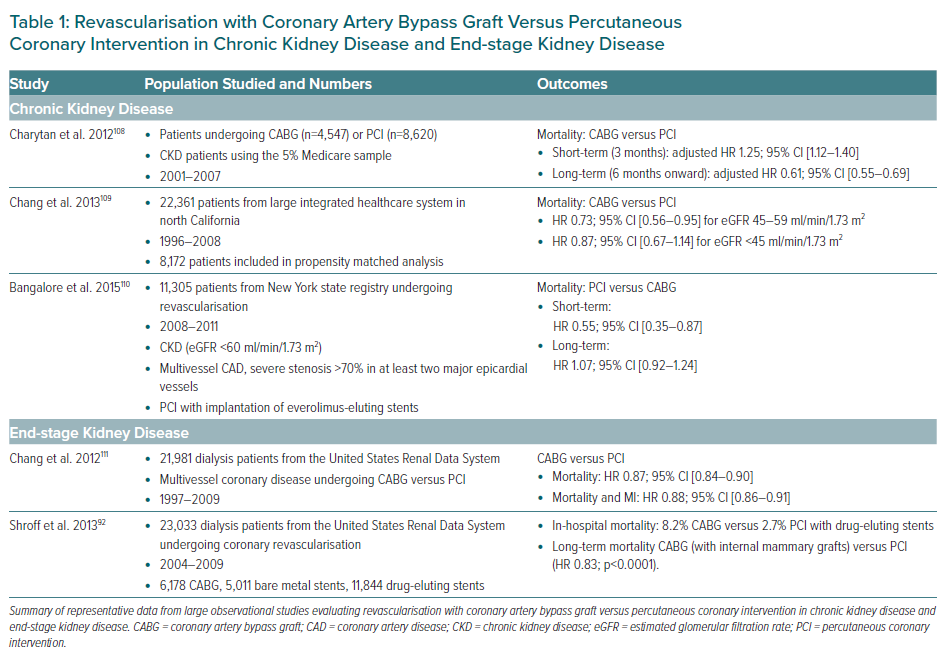
A randomised study of historical importance by Manske et al. first addressed the vexing issue of revascularisation in 151 insulin-dependent diabetic patients with CKD who underwent coronary angiography for renal transplantation evaluation.85 In this small cohort of 26 patients who were randomised to medical therapy (calcium channel blocker plus aspirin) versus revascularisation, a significant survival benefit was noted with revascularisation compared with medical therapy. Since then, but prior to the publication of ISCHEMIA-CKD, a plethora of observational registries have compared the best modality for revascularisation in CKD/ESKD. In general, in this high-risk population, coronary artery bypass graft (CABG) is associated with higher in-hospital and short-term mortality rates than PCI, whereas in the long-term, CABG is associated with improved survival. A summary of published observational evidence pertaining to outcomes with surgical versus percutaneous revascularisation in CKD/ESKD is presented in Table 1. Several large systemic meta-analyses have also been performed to further consolidate data obtained from registries. These meta-analyses have consistently shown a survival advantage of CABG compared with PCI in long-term follow-up among patients with moderate and severe CKD.86–88
Several special considerations deserve specific mention. The context of revascularisation needs to be factored into decision-making, but in general, there is a dearth of specific information in this regard. In ESKD patients on dialysis, it was shown that CABG (versus PCI) was associated with higher long-term survival in the context of ACS, but had equivalent outcomes in the absence of ACS.89
Similarly, a study of Medicare beneficiaries evaluated the comparative effectiveness of different revascularisation modalities among 34,385 individuals with CKD.90 In high-risk patients (those presenting with ACS), revascularisation with CABG and PCI relative to medical therapy were associated with lower hazards of mortality, but not in low-risk patients. Finally, among 13,085 patients on dialysis undergoing CABG, off-pump CABG was associated with a lower risk of in-hospital mortality from any cause (HR 0.92; 95% CI [0.86–0.99]).91 However, the survival difference was no longer significant after 2 years, suggesting that the choice of surgical technique should be deferred to the local expertise and preference of the surgeon.
Ultimately, only a randomised clinical trial would be able to accurately discern differences in outcomes between CABG versus PCI in CKD/ESKD patients. ISCHEMIA-CKD is the largest randomised clinical trial to date examining the optimal therapy for chronic CAD in patients with CKD stages G4–5D.38 There was no benefit to an early invasive strategy of coronary revascularisation combined with optimised guideline-directed medical therapy (GDMT) compared with GDMT alone (with revascularisation reserved for GDMT failure). Several important points can be garnered from this significant clinical trial. The paradigm of non-invasive testing to identify clinically important coronary stenosis in a largely asymptomatic population requires re-evaluation. Just over half of those who underwent ICA were identified as having lesions that were amenable to intervention, despite meeting the entry criteria of moderate or severe ischaemia on non-invasive stress testing, revealing a disconnect between stress testing and ICA results. Also, GDMT was well-tolerated, and goals were achievable; maximising current risk reduction strategies may maximise benefits among patients with longer life-expectancy or candidates for transplantation. ISCHEMIA-CKD has revealed the importance of specific hypothesis testing of CV treatment strategies in this high-risk population.
Proposed Clinical Approach to Periprocedural Management in Patients Needing Coronary Revascularisation
Due to the high-risk nature of this population, coronary revascularisation requires meticulous planning to reduce attendant risks of CV and renal decompensation. We recommend a heart–kidney team-based approach with careful consideration of the following variables.
Choice of Coronary Artery Bypass Graft Versus Percutaneous Coronary Intervention
First, a typical ‘heart team’ approach comprises input from interventional cardiology and cardiac surgeons for these considerations, but a ‘heart–kidney’ team approach is necessitated in this population to individualise the decision after careful deliberation of the trade-offs involved. We suggest using a ‘shared decision-making’ construct with the patient, including a detailed, carefully weighed discussion of short-term/in-hospital mortality versus long-term risks/benefits of CABG versus PCI in a shared decision-making format with participation of the cardiologist and nephrologist, while actively factoring in the patient’s preferences. The baseline high competing risk of all-cause mortality with worsening CKD/ESKD needs to be reconciled.
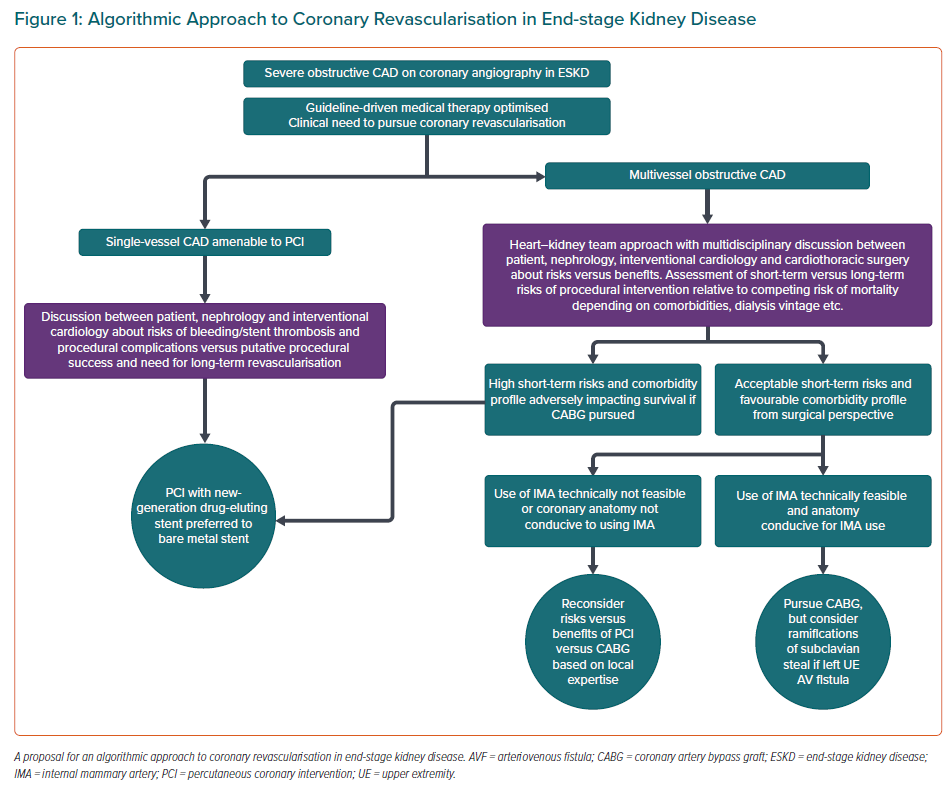
Second, in patients with ESKD on haemodialysis, we propose an algorithmic approach outlined in Figure 1 that encapsulates the main tenets of learnings from observational evidence. It does bear emphasis that the use of internal mammary artery grafts has been consistently shown to independently confer a survival advantage among ESKD patients undergoing surgical revascularisation.91,92
Third, among patients with CKD, additional input from the nephrologist is extremely valuable. Particularly among dialysis patients, nephrologists typically have a long-standing, continuity relationship with the patient, and can provide very meaningful input pertaining to outlook/preferences, long-term prognosis, and nuances regarding dialysis access and metabolic management.
Fourth, apart from the usual consideration pertaining to coronary anatomy and LV dysfunction, specific considerations of importance in patients with CKD include manifestations of deranged calcium/phosphorus/parathyroid metabolism, such as accompanying haemodynamically significant valvular disease (mitral annular calcification causing mitral stenosis/calcific aortic stenosis) or the presence of a ‘porcelain’ aorta. These factors could specifically impact the choice of a surgical versus percutaneous approach to coronary revascularisation.
Fifth, regarding the bleeding risk, short-term bleeding risk is increased in patients with CKD.93 More recently, a lower risk of transfusion requirement with a transradial versus transfemoral approach has been shown among patients with CKD.94 Relaying the risks of bleeding with anticoagulation related to the acute procedure, as well as long-term anticoagulation if PCI is required, is important during shared decision-making. Ensuring appropriate monitoring and minimising important drug–drug interactions that can enhance bleeding (e.g. non-steroidal anti-inflammatory agents with antiplatelet drugs) are critical to minimising bleeding risk over the long term. Further studies on minimising duration of antiplatelet therapy post-PCI may also further lower bleeding risk in the CKD population.
Sixth, radial access for PCI, as mentioned, has relative merits in terms of lower risk of dialysis, and lower postprocedural transfusion requirements.94 However, a subset of patients with CKD stage G5D will require the radial artery (typically of the non-dominant arm; dependent on the suitability of the veins) for radiocephalic arteriovenous fistula creation. Whether the use of the radial artery approach affects its future usability as an inflow or the survival of an existing radiocephalic fistula should merit specific discussion. These outcomes should be tracked in future studies.
Assessing and Mitigating the Risk of Acute Kidney Injury
Acute kidney injury (AKI) can occur due to many different contributors in patients with concomitant CAD and CKD, including haemodynamic perturbations from congestion and low cardiac output/cardiogenic shock, cardiopulmonary pump run and inflammatory milieu of the membrane, use of intra-aortic balloon pump and extracorporeal support, atheroembolic phenomenon, and so on. The importance of mitigation of AKI risk is not limited to the acute hospitalisation or periprocedural period. There is a real risk of new/progressive CKD in patients who suffer AKI – this been demonstrated among patients undergoing ICA with subsequent contrast-associated AKI.95,96 Several validated risk calculators are easily available to prospectively assess the risk of AKI following PCI/CABG.97,98 Such risk calculators can provide clinicians guidance pertaining to estimated risk, to best plan management strategies.
Meersch et al. demonstrated in a single-centre trial that implementation of a ‘KDIGO-bundle’ versus standard care in high-risk patients undergoing CABG reduced the risk of AKI significantly (55% versus 72%, p<0.004).99 This ‘KDIGO-bundle’ includes avoiding nephrotoxic agents, discontinuing angiotensin-converting enzyme inhibitors/angiotensin II antagonists for the preceding 48 hours, close monitoring of creatinine/urine output, avoiding hyperglycaemia and radiocontrast agents, and close monitoring to optimise volume status/haemodynamic parameters.100 Furthermore, this study demonstrated the salutary role of urinary biomarkers in the early detection of AKI. Using biomarkers to assess high-risk postoperative patients can aid in implementing early risk mitigating measures (e.g. optimisation of volume and haemodynamic status, assuring appropriate antibiotic levels and minimising the use of potentially nephrotoxic agents).
Risk of Contrast-induced Acute Kidney Injury
Among patients with pre-dialysis CKD, careful prospective assessment of the risk of contrast-induced (CI) AKI is paramount. Limiting the use of iodinated contrast medium to the smallest possible volume, including consideration of ‘staged’ procedures requiring high volumes of contrast. It is increasingly recognised that risks associated with iodinated contrast medium are often overestimated.101
An individualised approach guided by left ventricular end-diastolic pressure has been shown to lead to a substantial reduction in CI-AKI.102 Also, among patients at high-risk for AKI, there was no benefit to intravenous sodium bicarbonate compared with normal saline, or of oral acetylcysteine compared with placebo in preventing of CI-AKI.103 This finding was further substantiated specifically among patients with CKD.104 Thus, hydration with intravenous normal saline or orally remains the cornerstone intervention for preventing CI-AKI.
Avoiding Nephrotoxic Agents
Apart from iodinated contrast medium, there are several nephrotoxic agents that could contribute to AKI in the context of coronary revascularisation. It is prudent to avoid the ‘triple whammy’ of renin–angiotensin aldosterone blockers, diuretics and non-steroidal inflammatory agents.101 It is further recommend to include a clinical pharmacist in the team for drug stewardship.
Dialysis Management
Occasionally renal replacement therapy (RRT) becomes necessary in the context of AKI in CKD. The KDIGO group has provided detailed guiding principles for the use of RRT in the context of AKI, including vascular access, dialysis prescription and management of anticoagulation including in the setting of extracorporeal support. It is important to highlight that typically, in haemodynamically unstable situations continuous rather than intermittent RRT is recommended; but the selection of modalities is dependent upon several clinical variables and requires careful planning by the heart–kidney team. Perioperative risks of patients with CKD 5D (on chronic dialysis) are exceedingly high, necessitating astute perioperative planning of dialysis.
For patients on chronic peritoneal dialysis (PD), it is preferred to continue PD post-CABG, since survival is comparable to haemodialysis.105 Furthermore, haemodynamic stability with PD is better than intermittent haemodialysis, and thus PD can be considered even in haemodynamically unstable patients post-CABG.106 However, fluid and metabolic needs may sometimes overwhelm the clearance capabilities of the peritoneal membrane limiting PD. In particular, among patients undergoing high-risk CABG, if haemodynamically unstable or continuous RRT is expected postprocedure, clinicians should be aware that an arteriovenous fistula/graft may not be able to provide continuous dialysis, and PD may not be adequate in cases of marked hyperkalaemia. An intentional pre-procedural discussion with the patient and the nephrologist regarding post-CABG dialysis modality, and any anticipated need for temporary dialysis access placement, may circumvent the need for hurried access placement postprocedure.
Goal-directed Fluid Therapy and Haemodynamic Management
A goal-directed fluid management strategy is suggested rather than an informal approach to fluid management to preventing AKI, which can be critical among patients undergoing complex coronary revascularisation.97 The use of pulmonary artery catheters to assess volume status and cardiac output may be helpful in selected subsets of patients with severe ventricular dysfunction, accompanying valvular disease and significant pulmonary hypertension.107
Conclusion
Patients with CKD/ESKD and concomitant CAD constitute a high-risk population with unique epidemiological and pathophysiological characteristics, as well as nuances pertaining to non-invasive risk assessment, medical management and coronary revascularisation. A collaborative heart–kidney team-based approach is imperative for critical management decisions for this patient population, especially when pursuing coronary revascularisation.